Thiopeptide Engineering: A Multidisciplinary Effort towards Future Drugs
Xavier Just-Baringo
Institute for Research in Biomedicine, Barcelona Science Park, University of Barcelona, Baldiri Reixac 10, 08028 Barcelona (Spain) http://www.pcb.ub.edu/fama/htm/home.htm
CIBER-BBN, Networking Centre on Bioengineering Biomaterials and Nanomedicine, 08028 Barcelona (Spain)
Search for more papers by this authorProf. Fernando Albericio
Institute for Research in Biomedicine, Barcelona Science Park, University of Barcelona, Baldiri Reixac 10, 08028 Barcelona (Spain) http://www.pcb.ub.edu/fama/htm/home.htm
CIBER-BBN, Networking Centre on Bioengineering Biomaterials and Nanomedicine, 08028 Barcelona (Spain)
Department of Organic Chemistry, University of Barcelona, 08028 Barcelona (Spain)
School of Chemistry and Physics, University of KwaZulu-Natal, 4000-Durban (South Africa)
Search for more papers by this authorCorresponding Author
Prof. Mercedes Álvarez
Institute for Research in Biomedicine, Barcelona Science Park, University of Barcelona, Baldiri Reixac 10, 08028 Barcelona (Spain) http://www.pcb.ub.edu/fama/htm/home.htm
CIBER-BBN, Networking Centre on Bioengineering Biomaterials and Nanomedicine, 08028 Barcelona (Spain)
Laboratory of Organic Chemistry, Faculty of Pharmacy, University of Barcelona, 08028 Barcelona (Spain)
Institute for Research in Biomedicine, Barcelona Science Park, University of Barcelona, Baldiri Reixac 10, 08028 Barcelona (Spain) http://www.pcb.ub.edu/fama/htm/home.htmSearch for more papers by this authorXavier Just-Baringo
Institute for Research in Biomedicine, Barcelona Science Park, University of Barcelona, Baldiri Reixac 10, 08028 Barcelona (Spain) http://www.pcb.ub.edu/fama/htm/home.htm
CIBER-BBN, Networking Centre on Bioengineering Biomaterials and Nanomedicine, 08028 Barcelona (Spain)
Search for more papers by this authorProf. Fernando Albericio
Institute for Research in Biomedicine, Barcelona Science Park, University of Barcelona, Baldiri Reixac 10, 08028 Barcelona (Spain) http://www.pcb.ub.edu/fama/htm/home.htm
CIBER-BBN, Networking Centre on Bioengineering Biomaterials and Nanomedicine, 08028 Barcelona (Spain)
Department of Organic Chemistry, University of Barcelona, 08028 Barcelona (Spain)
School of Chemistry and Physics, University of KwaZulu-Natal, 4000-Durban (South Africa)
Search for more papers by this authorCorresponding Author
Prof. Mercedes Álvarez
Institute for Research in Biomedicine, Barcelona Science Park, University of Barcelona, Baldiri Reixac 10, 08028 Barcelona (Spain) http://www.pcb.ub.edu/fama/htm/home.htm
CIBER-BBN, Networking Centre on Bioengineering Biomaterials and Nanomedicine, 08028 Barcelona (Spain)
Laboratory of Organic Chemistry, Faculty of Pharmacy, University of Barcelona, 08028 Barcelona (Spain)
Institute for Research in Biomedicine, Barcelona Science Park, University of Barcelona, Baldiri Reixac 10, 08028 Barcelona (Spain) http://www.pcb.ub.edu/fama/htm/home.htmSearch for more papers by this authorGraphical Abstract
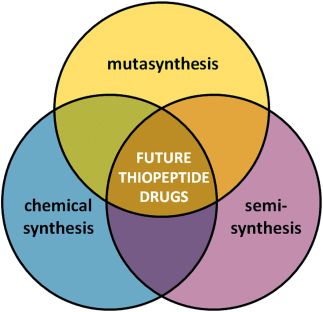
Making a complement: Modification of the structure of thiopeptides has produced numerous analogues that overcome some of their inherent limitations. The combined use of chemical synthesis, semisynthesis, and biosynthetic pathway engineering will allow the development of future thiopeptide-based drugs.
Abstract
The recent development of thiopeptide analogues of antibiotics has allowed some of the limitations inherent to these naturally occurring substances to be overcome. Chemical synthesis, semisynthetic derivatization, and engineering of the biosynthetic pathway have independently led to complementary modifications of various thiopeptides. Some of the new substances have displayed improved profiles, not only as antibiotics, but also as antiplasmodial and anticancer drugs. The design of novel molecules based on the thiopeptide scaffold appears to be the only strategy to exploit the high potential they have shown in vitro. Herein we present the most relevant achievements in the production of thiopeptide analogues and also discuss the way the different approaches might be combined in a multidisciplinary strategy to produce more sophisticated structures.
References
- 1M. C. Bagley, J. W. Dale, E. A. Merritt, X. Xiong, Chem. Rev. 2005, 105, 685–714.
- 2R. A. Hughes, C. J. Moody, Angew. Chem. 2007, 119, 8076–8101;
10.1002/ange.200700728 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 7930–7954.
- 3H. A. Kirst, Expert Opin. Drug Discovery 2013, 8, 479–493.
- 4K. C. Nicolaou, B. S. Safina, M. Zak, A. A. Estrada, S. H. Lee, Angew. Chem. 2004, 116, 5197–5202;
10.1002/ange.200461340 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 5087–5092.
- 5K. C. Nicolaou, M. Zak, B. S. Safina, S. H. Lee, A. A. Estrada, Angew. Chem. 2004, 116, 5202–5207;
10.1002/ange.200461341 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 5092–5097.
- 6H. M. Müller, O. Delgado, T. Bach, Angew. Chem. 2007, 119, 4855–4858; Angew. Chem. Int. Ed. 2007, 46, 4771–4774.
- 7D. Lefranc, M. A. Ciufolini, Angew. Chem. 2009, 121, 4262–4265;
10.1002/ange.200900621 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 4198–4201.
- 8R. A. Hughes, S. P. Thompson, L. Alcaraz, C. J. Moody, J. Am. Chem. Soc. 2005, 127, 15644–15651.
- 9V. S. Aulakh, M. A. Ciufolini, J. Am. Chem. Soc. 2011, 133, 5900–5904.
- 10X. Just-Baringo, P. Bruno, L. K. Ottesen, L. M. Cañedo, F. Albericio, M. Álvarez, Angew. Chem. 2013, 125, 7972–7975;
10.1002/ange.201302372 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 7818–7821.
- 11J.-Y. Lu, M. Riedrich, K. P. Wojtas, H.-D. Arndt, Synthesis 2013, 45, 1300–1311.
- 12H.-D. Arndt, S. Schoof, J.-Y. Lu, Angew. Chem. 2009, 121, 6900–6904;
10.1002/ange.200901808 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 6770–6773.
- 13C. Li, W. L. Kelly, Nat. Prod. Rep. 2010, 27, 153–164.
- 14J. Vandeputte, J. D. Dutcher, Antibiot. Ann. 1955, 3, 560–561.
- 15J. E. Leet, H. A. Ax, D. R. Gustavson, D. M. Brown, L. Turner, K. Brown, W. Li, K. S. Lam, Nocathiacin Antibiotics, U.S. Patent US 6218398 B1, 2001.
- 16J. Shoji, H. Hinoo, Y. Wakisaka, K. Koizumi, M. Mayama, S. Matsuura, K. Matsumoto, J. Antibiot. 1976, 29, 366–374.
- 17E. Selva, G. Beretta, N. Montanini, G. S. Saddler, L. Gastaldo, P. Ferrari, R. Lorenzzetti, P. Landini, F. Ripamonti, B. P. Goldstein, M. Berti, L. Montanaro, M. Denaro, J. Antibiot. 1991, 44, 693–701.
- 18R. P. Morris, J. A. Leeds, H. U. Naegeli, L. Oberer, K. Memmert, E. Weber, M. J. LaMarche, C. N. Parker, N. Burrer, S. Esterow, A. E. Hein, E. K. Schmitt, P. Krastel, J. Am. Chem. Soc. 2009, 131, 5946–5955.
- 19K. Engelhardt, K. F. Degnes, M. Kemmler, H. Bredholt, E. Fjaervik, G. Klinkenberg, H. Sletta, T. E. Ellingsen, S. B. Zotchev, Appl. Environ. Microbiol. 2010, 76, 4969–4976.
- 20H. Abe, K. Katsuhiko, S. Yoshinori, K. Mitsuaki, Tetrahedron Lett. 1988, 29, 1401–1404.
- 21J. Thompson, E. Cundliffe, M. Stark, Eur. J. Biochem. 1979, 98, 261–265.
- 22J. Thompson, E. Cundliffe, Biochimie 1991, 73, 1131–1135.
- 23B. T. Porse, I. Leviev, A. S. Mankin, R. A. Garrett, J. Mol. Biol. 1998, 276, 391–404.
- 24C. L. Myers, P. C. Hang, G. Ng, J. Yuen, J. F. Honek, Bioorg. Med. Chem. Lett. 2010, 18, 4231–4237.
- 25F. von Nussbaum, M. Brands, B. Hinzen, S. Weigand, D. Häbich, Angew. Chem. 2006, 118, 5194–5254;
10.1002/ange.200600350 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 5072–5129.
- 26K. C. Nicolaou, M. Nevalainen, M. Zak, S. Bulat, M. Bella, B. S. Safina, Angew. Chem. 2003, 115, 3540–3546;
10.1002/ange.200351745 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 3418–3424.
- 27K. C. Nicolaou, M. Zak, S. Rahimipour, A. A. Estrada, S. H. Lee, A. O’Brate, P. Giannakakou, M. R. Ghadiri, J. Am. Chem. Soc. 2005, 127, 15042–15044.
- 28K. C. Nicolaou, B. Zou, D. H. Dethe, D. B. Li, D. Y.-K. Chen, Angew. Chem. 2006, 118, 7950–7956;
10.1002/ange.200602798 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 7786–7792.
- 29A. L. Starosta, H. Qin, A. Mikolajka, G. Y. C. Leung, K. Schwinghammer, K. C. Nicolaou, D. Y.-K. Chen, B. S. Cooperman, D. N. Wilson, Chem. Biol. 2009, 16, 1087–1096.
- 30J. Bower, M. Drysdale, R. Hebdon, A. Jordan, G. Lentzen, N. Matassova, A. Murchie, J. Powles, S. Roughley, Bioorg. Med. Chem. Lett. 2003, 13, 2455–2458.
- 31B. N. Naidu, W. Li, M. E. Sorenson, T. P. Connolly, J. A. Wichtowski, Y. Zhang, O. K. Kim, J. D. Matiskella, K. S. Lam, J. J. Bronson, Y. Ueda, Tetrahedron Lett. 2004, 45, 1059–1063.
- 32B. N. Naidu, M. E. Sorenson, Y. Zhang, O. K. Kim, J. D. Matiskella, J. A. Wichtowski, T. P. Connolly, W. Li, K. S. Lam, J. J. Bronson, M. J. Pucci, J. M. Clark, Y. Ueda, Bioorg. Med. Chem. Lett. 2004, 14, 5573–5577.
- 33B. N. Naidu, M. E. Sorenson, J. J. Bronson, M. J. Pucci, J. M. Clark, Y. Ueda, Bioorg. Med. Chem. Lett. 2005, 15, 2069–2072.
- 34B. N. Naidu, W. Li, K. S. Lam, M. E. Sorenson, J. A. Wichtowski, T. P. Connolly, Y. Ueda, J. J. Bronson, Y. Zhang, O. K. Kim, Water Soluble Thiazolyl Peptide Derivatives, U.S. Patent US2002065219A1, 2002.
- 35B. N. Naidu, M. E. Sorenson, J. D. Matiskella, W. Li, J. B. Sausker, Y. Zhang, T. P. Connolly, K. S. Lam, J. J. Bronson, M. J. Pucci, H. Yang, Y. Ueda, Bioorg. Med. Chem. Lett. 2006, 16, 3545–3549.
- 36A. Regueiro-Ren, Y. Ueda, J. Org. Chem. 2002, 67, 8699–8702.
- 37T. P. Connolly, A. Regueiro-Ren, J. E. Leet, D. M. Springer, J. Goodrich, X. S. Huang, M. J. Pucci, J. M. Clark, J. J. Bronson, Y. Ueda, J. Nat. Prod. 2005, 68, 550–553.
- 38L. Xu, A. K. Farthing, Y.-J. Shi, P. T. Meinke, K. Liu, J. Org. Chem. 2007, 72, 7447–7450.
- 39L. Xu, A. K. Farthing, J. F. Dropinski, P. T. Meinke, C. McCallum, P. S. Leavitt, E. J. Hickey, L. Colwell, J. Barrett, K. Liu, Bioorg. Med. Chem. Lett. 2009, 19, 3531–3535.
- 40P. Hrnciar, Y. Ueda, S. Huang, J. E. Leet, J. J. Bronson, J. Org. Chem. 2002, 67, 8789–8793.
- 41M. J. Pucci, J. J. Bronson, J. F. Barrett, K. L. Denbleyker, L. F. Discotto, C. Joan, J. C. Fung-Tomc, Y. Ueda, Antimicrob. Agents Chemother. 2004, 48, 3697–3701.
- 42A. Regueiro-Ren, B. N. Naidu, X. Zheng, T. W. Hudyma, T. P. Connolly, J. D. Matiskella, Y. Zhang, O. K. Kim, M. E. Sorenson, M. Pucci, J. Clark, J. J. Bronson, Y. Ueda, Bioorg. Med. Chem. Lett. 2004, 14, 171–175.
- 43B. N. Naidu, M. E. Sorenson, T. Hudyma, X. Zheng, Y. Zhang, J. J. Bronson, M. J. Pucci, J. M. Clark, Y. Ueda, Bioorg. Med. Chem. Lett. 2004, 14, 3743–3746.
- 44J. Golik, H. S. L. Wong, S. H. Chcn, T. W. Doyle, J. J. K. Wright, J. Knipe, W. C. Rose, A. M. Casazza, D. M. Vyas, Bioorg. Med. Chem. Lett. 1996, 6, 1837–1842.
- 45T. Tojo, T. Hanadate, A. Okada, J. Kazami, S. Takeda, M. Shibazaki, Preparation of Cyclic Thiopeptides (QN3323-A Derivatives) and Their Pharmaceutical Compositions for Multidrug-Resistant Bacteria, U.S. Patent J2008115165A, 2008.
- 46K. Junichi, O. Takashi, W. Masato, K. Kazuma, Y. Takashi, T. Kaniaki, Preparation of O-Acyl-QN3323-A Oxime Thiopeptide Compounds as Antibacterial Agents, U.S. Patent JP2009018991A, 2009.
- 47M. J. LaMarche, J. A. Leeds, J. Dzink-Fox, E. Gangl, P. Krastel, G. Neckermann, D. Palestrant, M. A. Patane, E. M. Rann, S. Tiamfook, D. H. Yu, J. Med. Chem. 2012, 55, 6934–6941.
- 48P. Ferrari, L. Colombo, S. Stella, E. Selva, L. F. Zerilli, J. Antibiot. 1995, 48, 1304–1311.
- 49P. Tavecchia, P. Gentili, M. Kurz, C. Sottani, R. Bonfichi, E. Selva, S. Lociuro, E. Restelli, R. Ciabatti, Tetrahedron 1995, 51, 4867–4890.
- 50P. Tavecchia, M. Kurz, L. Colombo, R. Bonfichi, E. Selva, S. Lociuro, E. Marzorati, R. Ciabatti, Tetrahedron 1996, 52, 8763–8774.
- 51S. Lociuro, P. Tavecchia, E. Marzorati, P. Landini, B. P. Goldstein, M. Denarod, R. Ciabatti, J. Antibiot. 1997, 50, 344–349.
- 52A. Malabarba, M. Cavaleri, G. Mosconi, D. Jabes, G. Romano, Use of Amide Derivative of GE 2270 Factor a3 for the Treatment of Acne, U.S. Patent WO 03105881, 2007.
- 53J. Clough, S. Chen, E. M. Gordon, C. Hackbarth, S. Lam, J. Trias, R. J. White, G. Candiani, S. Donadio, G. Romanò, R. Ciabatti, J. W. Jacobs, Bioorg. Med. Chem. Lett. 2003, 13, 3409–3414.
- 54M. J. LaMarche, J. A. Leeds, J. Dzink-Fox, K. Gunderson, P. Krastel, K. Memmert, M. A. Patane, E. M. Rann, E. Schmitt, S. Tiamfook, B. Wang, J. Med. Chem. 2011, 54, 2517–2521.
- 55M. J. LaMarche, J. A. Leeds, J. Dzink-Fox, S. Mullin, M. A. Patane, E. M. Rann, S. Tiamfook, Bioorg. Med. Chem. Lett. 2011, 21, 3210–3215.
- 56M. J. LaMarche, J. Leeds, K. Amaral, J. Brewer, S. Bushell, J. Dewhurst, J. Dzink-Fox, E. Gangl, J. Goldovitz, A. Jain, S. Mullin, G. Neckermann, C. Osborne, D. Palestrant, M. A. Patane, E. M. Rann, M. Sachdeva, J. Shao, S. Tiamfbok, L. Whitehead, D. H. Yu, J. Med. Chem. 2011, 54, 8099–8109.
- 57M. J. LaMarche, S. Bushell, M. A. Patane, L. Whitehead, Aminothiazoles and Their Uses, U.S. Patent WO2007142986, 2010.
- 58J. A. Leeds, M. J. LaMarche, J. T. Brewer, S. M. Bushell, G. Deng, J. M. Dewhurst, J. Dzink-Fox, E. Gangl, A. Jain, L. Lee, M. Lilly, K. Manni, S. Mullin, G. Neckermann, C. Osborne, D. Palestrant, M. A. Patane, A. Raimondi, S. Ranjitkar, E. M. Rann, M. Sachdeva, J. Shao, S. Tiamfook, L. Whitehead, D. Yu, Antimicrob. Agents Chemother. 2011, 55, 5277–5283.
- 59M. J. LaMarche, J. A. Leeds, A. Amaral, J. T. Brewer, S. M. Bushell, G. Deng, J. M. Dewhurst, J. Ding, J. Dzink-fox, G. Gamber, A. Jain, K. Lee, L. Lee, T. Lister, D. McKenney, S. Mullin, C. Osborne, D. Palestrant, M. A. Patane, E. M. Rann, M. Sachdeva, J. Shao, S. Tiamfook, A. Trzasko, L. Whitehead, A. Yifru, D. H. Yu, W. L. Yan, Q. M. Zhu, J. Med. Chem. 2012, 55, 2376–2387.
- 60A. Trzasko, J. A. Leeds, J. Praestgaard, M. J. LaMarche, D. McKenney, Antimicrob. Agents Chemother. 2012, 56, 4459–4462.
- 61L. S. L. Ting, J. Praestgaard, N. Grunenberg, J. C. Yang, J. A. Leeds, P. Pertel, Antimicrob. Agents Chemother. 2012, 56, 5946–5951.
- 62J. A. Leeds, M. Sachdeva, S. Mullin, J. Dzink-Fox, M. J. LaMarche, Antimicrob. Agents Chemother. 2012, 56, 4463–4465.
- 63S. S. Myatt, E. W.-F. Lam, Nat. Rev. Cancer 2007, 7, 847–859.
- 64N. S. Hegde, D. A. Sanders, R. Rodriguez, S. Balasubramanian, Nat. Chem. 2011, 3, 725–731.
- 65S. Schoof, S. Baumann, B. Ellinger, H.-D. Arndt, ChemBioChem 2009, 10, 242–245.
- 66S. Schoof, G. Pradel, M. N. Aminake, B. Ellinger, S. Baumann, M. Potowski, Y. Najajreh, M. Kirschner, H.-D. Arndt, Angew. Chem. 2010, 122, 3389–3393;
10.1002/ange.200906988 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 3317–3321.
- 67S. Baumann, S. Schoof, M. Bolten, C. Haering, M. Takagi, K. Shin-ya, H.-D. Arndt, J. Am. Chem. Soc. 2010, 132, 6973–6981.
- 68S. Schoof, H.-D. Arndt, Chem. Commun. 2009, 7113–7115.
- 69B. Pandit, U. Bhat, A. L. Gartel, Cancer Biol. Ther. 2011, 11, 43–47.
- 70H. R. A. Jonker, S. Baumann, A. Wolf, S. Schoof, F. Hiller, K. W. Schulte, K. N. Kirschner, H. Schwalbe, H.-D. Arndt, Angew. Chem. 2011, 123, 3366–3370; Angew. Chem. Int. Ed. 2011, 50, 3308–3312.
- 71C. J. Schofield, J. E. Baldwin, M. F. Byford, I. Clifton, J. Hajdu, C. Hensgens, P. Roach, Curr. Opin. Struct. Biol. 1997, 7, 857–864.
- 72Q. Zhang, W. Liu, Nat. Prod. Rep. 2013, 30, 218–226.
- 73F. Zhang, W. L. Kelly, In Vivo Production of Thiopeptide Variants, Elsevier, San Diego, 2012.
10.1016/B978-0-12-394291-3.00022-8 Google Scholar
- 74M. G. Acker, A. A. Bowers, C. T. Walsh, J. Am. Chem. Soc. 2009, 131, 17563–17565.
- 75A. A. Bowers, M. G. Acker, A. Koglin, C. T. Walsh, J. Am. Chem. Soc. 2010, 132, 7519–7527.
- 76T. S. Young, C. T. Walsh, Proc. Natl. Acad. Sci. USA 2011, 108, 13053–13058.
- 77C. Li, F. Zhang, W. L. Kelly, Mol. BioSyst. 2011, 7, 82–90.
- 78C. Li, F. Zhang, W. L. Kelly, Chem. Commun. 2012, 48, 558–560.
- 79T. S. Young, P. C. Dorrestein, C. T. Walsh, Chem. Biol. 2012, 19, 1600–1610.
- 80A. A. Bowers, M. G. Acker, T. S. Young, C. T. Walsh, J. Am. Chem. Soc. 2012, 134, 10313–10316.
- 81A. A. Bowers, C. T. Walsh, M. G. Acker, J. Am. Chem. Soc. 2010, 132, 12182–12184.
- 82W. L. Kelly, L. Pan, C. Li, J. Am. Chem. Soc. 2009, 131, 4327–4334.
- 83R. Liao, W. Liu, J. Am. Chem. Soc. 2011, 133, 2852–2855.
- 84Y. Yu, H. Guo, Q. Zhang, L. Duan, Y. Ding, R. Liao, C. Lei, B. Shen, W. Liu, J. Am. Chem. Soc. 2010, 132, 16324–16326.
- 85Y. Yu, L. Duan, Q. Zhang, R. Liao, Y. Ding, H. Pan, E. Wendt, G. Tang, B. Shen, W. Liu, ACS Chem. Biol. 2009, 4, 855–864.
- 86Q. Zhang, Y. Li, D. Chen, Y. Yu, L. Duan, B. Shen, W. Liu, Nat. Chem. Biol. 2011, 7, 154–160.
- 87Q. Zhang, D. Chen, J. Lin, R. Liao, W. Tong, Z. Xu, W. Liu, J. Biol. Chem. 2011, 286, 21287–21294.
- 88L. Duan, S. Wang, R. Liao, W. Liu, Chem. Biol. 2012, 19, 443–448.
- 89M. Wei, S. Wang, Y. Fang, Y. Chen, Bioresour. Technol. 2010, 101, 3617–3622.
- 90A. Kirschning, F. Hahn, Angew. Chem. 2012, 124, 4086–4096;
10.1002/ange.201107386 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 4012–4022.
- 91After the submission of this review, the first library of fully synthetic thiopeptide analogues was reported: X. Just-Baringo, P. Bruno, C. Pitart, J. Vila, F. Albericio, M. Álvarez, J. Med. Chem. 2014, DOI: .