Highly Enantioselective Aza-Diels–Alder Reaction of 1-Azadienes with Enecarbamates Catalyzed by Chiral Phosphoric Acids†
Dr. Long He
Centre de Recherche de Gif, Institut de Chimie des Substances Naturelles, CNRS, 91198 Gif-sur-Yvette Cedex (France)
Search for more papers by this authorGregory Laurent
Centre de Recherche de Gif, Institut de Chimie des Substances Naturelles, CNRS, 91198 Gif-sur-Yvette Cedex (France)
Search for more papers by this authorDr. Pascal Retailleau
Centre de Recherche de Gif, Institut de Chimie des Substances Naturelles, CNRS, 91198 Gif-sur-Yvette Cedex (France)
Search for more papers by this authorDr. Benoît Folléas
Diverchim, ZAC du Moulin, 6 rue du Noyer, 95700 Roissy-en-France (France)
Search for more papers by this authorDr. Jean-Louis Brayer
Diverchim, ZAC du Moulin, 6 rue du Noyer, 95700 Roissy-en-France (France)
Search for more papers by this authorCorresponding Author
Dr. Géraldine Masson
Centre de Recherche de Gif, Institut de Chimie des Substances Naturelles, CNRS, 91198 Gif-sur-Yvette Cedex (France)
Centre de Recherche de Gif, Institut de Chimie des Substances Naturelles, CNRS, 91198 Gif-sur-Yvette Cedex (France)Search for more papers by this authorDr. Long He
Centre de Recherche de Gif, Institut de Chimie des Substances Naturelles, CNRS, 91198 Gif-sur-Yvette Cedex (France)
Search for more papers by this authorGregory Laurent
Centre de Recherche de Gif, Institut de Chimie des Substances Naturelles, CNRS, 91198 Gif-sur-Yvette Cedex (France)
Search for more papers by this authorDr. Pascal Retailleau
Centre de Recherche de Gif, Institut de Chimie des Substances Naturelles, CNRS, 91198 Gif-sur-Yvette Cedex (France)
Search for more papers by this authorDr. Benoît Folléas
Diverchim, ZAC du Moulin, 6 rue du Noyer, 95700 Roissy-en-France (France)
Search for more papers by this authorDr. Jean-Louis Brayer
Diverchim, ZAC du Moulin, 6 rue du Noyer, 95700 Roissy-en-France (France)
Search for more papers by this authorCorresponding Author
Dr. Géraldine Masson
Centre de Recherche de Gif, Institut de Chimie des Substances Naturelles, CNRS, 91198 Gif-sur-Yvette Cedex (France)
Centre de Recherche de Gif, Institut de Chimie des Substances Naturelles, CNRS, 91198 Gif-sur-Yvette Cedex (France)Search for more papers by this authorWe wish to thank the ICSN and CNRS for financial support and Pascal Retailleau in ICSN for the X-ray crystallographic analysis. H.L. also acknowledges ANR for post-doctoral fellowships. G.L. thanks Diverchim for a CIFRE grant.
Graphical Abstract
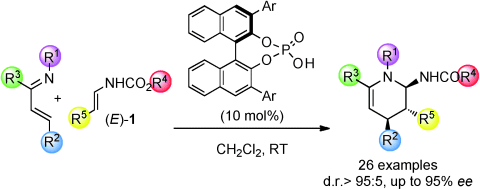
On demand: A highly enantio- and diastereoselective synthesis of 6-amino- trisubstituted tetrahydropyridine compounds has been developed through the inverse-electron-demand aza-Diels–Alder reaction of N-aryl α,β-unsaturated ketimines with enecarbamates (E)-1. Chiral phosphoric acid catalysts achieve simultaneous activation of both the 1-azadiene and dienophile partners.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201304969_sm_miscellaneous_information.pdf5.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. P. Michael in The Alkaloids, Vol. 55 (Ed.: ), Academic Press, San Diego, 2001;
- 1bP. D. Bailey, P. A. Millwood, P. D. Smith, Chem. Commun. 1998, 633–640;
- 1cY. Nishimura, T. Satoh, H. Adachi, S. Kondo, T. Takeuchi, M. Azetaka, H. Fukuyasu, Y. Lizuka, J. Med. Chem. 1997, 40, 2626–2633;
- 1dP. S. Watson, B. Jiang, B. Scott, Org. Lett. 2000, 2, 3679–3681;
- 1eS. Knapp, D. Zhao, Org. Lett. 2000, 2, 4037–4040;
- 1fE. Shitara, Y. Nishimura, K. Nerome, Y. Hiramoto, T. Takeuchi, Org. Lett. 2000, 2, 3837–3840;
- 1gN. Asano, R. J. Nash, R. J. Molyneux, G. W. J. Fleet, Tetrahedron: Asymmetry 2000, 11, 1645–1680;
- 1hV. H. Lillelund, H. H. Jensen, X. F. Liang, M. Bols, Chem. Rev. 2002, 102, 515–553;
- 1iM. Amat, N. Llor, J. Hidalgo, C. Escolano, J. Bosch, J. Org. Chem. 2003, 68, 1919–1928;
- 1jP. M. Weintraub, J. S. Sabol, J. M. Kane, D. R. Borcherding, Tetrahedron 2003, 59, 2953–2989;
- 1kM. G. P. Buffat, Tetrahedron 2004, 60, 1701–1729;
- 1lF.-X. Felpin, J. Lebreton, Curr. Org. Synth. 2004, 1, 83–109;
- 1mN. Zanatta, L. da S. Fernandes, F. M. Nachtigall, H. S. Coelho, S. S. Amaral, A. F. C. Flores, H. G. Bonacorso, M. A. P. Martins, Eur. J. Org. Chem. 2009, 1435–1444, and references therein;
- 1nX. G. Huang, A. Q. Zhang, D. L. Chen, Z. H. Jia, X. S. Li, Bioorg. Med. Chem. Lett. 2010, 20, 2859–2863.
- 2
- 2aX. Jiang, R. Wang, Chem. Rev. 2013, 113, 5515–5546;
- 2bG. Masson, C. Lalli, M. Benohoud, G. Dagousset, Chem. Soc. Rev. 2013, 42, 902–923;
- 2cP. R. Girling, T. Kiyoi, A. Whiting, Org. Biomol. Chem. 2011, 9, 3105–3121;
- 2dV. V. Kouznetsov, Tetrahedron 2009, 65, 2721–2750;
- 2eP. Buonora, J.-C. Olsen, T. Oh, Tetrahedron 2001, 57, 6099–6138.
- 3
- 3aM. E. Jung, J. J. Shapiro, J. Am. Chem. Soc. 1980, 102, 7862–7866; for selected review, see:
- 3bJ. K. Whitesell, M. A. Whitesell, Synthesis 1983, 517–536;
- 3cD. L. Boger, S. M. Weinreb, Hetero Diels–Alder Methodology in Organic Synthesis, Academic Press, San Diego, 1987, chap. 2, pp. 34–70; chap. 9, pp. 239–299, and references therein;
- 3dM. Behforouz, M. Ahmadian, Tetrahedron 2000, 56, 5259–5288;
- 3eB. Groenendaal, E. Ruijter, R. V. A. Orru, Chem. Commun. 2008, 5474–5489;
- 3fJ.-C. M. Monbaliu, K. G. R. Masschelein, C. V. Stevens, Chem. Soc. Rev. 2011, 40, 4708–4739.
- 4
- 4aY.-S. Cheng, A. T. Lupo, F. W. Fowler, J. Am. Chem. Soc. 1983, 105, 7696–7703;
- 4bY. C. Hwang, F. W. Fowler, J. Org. Chem. 1985, 50, 2719–2726;
- 4cN. J. Sisti, I. A. Motorina, M.-E. Tran Huu Dau, C. Riche, F. W. Fowler, D. S. Grierson, J. Org. Chem. 1996, 61, 3715–3728.
- 5
- 5aD. L. Boger, A. M. Kasper, J. Am. Chem. Soc. 1989, 111, 1517–1519;
- 5bD. L. Boger, W. L. Corbett, J. M. Wiggins, J. Org. Chem. 1990, 55, 2999–3000;
- 5cD. L. Boger, T. T. Curran, J. Org. Chem. 1990, 55, 5439–5442;
- 5dD. L. Boger, W. L. Corbett, T. T. Curran, A. M. Kasper, J. Am. Chem. Soc. 1991, 113, 1713–1729;
- 5eR. C. Clark, S. S. Pfeiffer, D. L. Boger, J. Am. Chem. Soc. 2006, 128, 2587–2593, and references therein.
- 6M. He, J. R. Struble, J. W. Bode, J. Am. Chem. Soc. 2006, 128, 8418–8420.
- 7
- 7aJ. Esquivias, R. Gómez Arrayás, J. C. Carretero, J. Am. Chem. Soc. 2007, 129, 1480–1481;
- 7bJ. Esquivias, I. Alonso, R. Gómez Arrayás, J. C. Carretero, Synthesis 2009, 113–126.
- 8
- 8aJ.-P. Wan, C. C. J. Loh, F. Pan, D. Enders, Chem. Commun. 2012, 48, 10049–10051;
- 8bB. Han, J.-L. Li, C. Ma, S.-J. Zhang, Y.-C. Chen, Angew. Chem. 2008, 120, 10119–10122; Angew. Chem. Int. Ed. 2008, 47, 9971–9974;
- 8cJ.-L. Li, T.-Y. Liu, Y.-C. Chen, Acc. Chem. Res. 2012, 45, 1491–1500;
- 8dJ.-L. Li, S.-L. Zhou, B. Han, L. Wu, Y.-C. Chen, Chem. Commun. 2010, 46, 2665–2667;
- 8eZ.-Q. He, B. Han, R. Li, L. Wu, Y.-C. Chen, Org. Biomol. Chem. 2010, 8, 755–757;
- 8fS.-L. Zhou, J.-L. Li, L. Dong, Y.-C. Chen, Org. Lett. 2011, 13, 5874–5877;
- 8gB. Han, Z.-Q. He, J.-L. Li, R. Li, K. Jiang, T.-Y. Liu, Y.-C. Chen, Angew. Chem. 2009, 121, 5582–5585; Angew. Chem. Int. Ed. 2009, 48, 5474–5477;
- 8hC. Simal, T. Lebl, A. M. Z. Slawin, A. D. Smith, Angew. Chem. 2012, 124, 3713–3717;
10.1002/ange.201109061 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 3653–3657;
- 8iX. Jiang, X. Shi, S. Wang, T. Sun, Y. Cao, R. Wang, Angew. Chem. 2012, 124, 2126–2129; Angew. Chem. Int. Ed. 2012, 51, 2084–2087;
- 8jY. Deng, L. Liu, R. G. Sarkisian, K. Wheeler, H. Wang, Z. Xu, Angew. Chem. 2013, 125, 3448–3452; Angew. Chem. Int. Ed. 2013, 52, 3364–3667;
- 8kZ. Chen, B. Wang, Z. Wang, G. Zhu, J. Sun, Angew. Chem. 2013, 125, 2081–2085; Angew. Chem. Int. Ed. 2013, 52, 2027–2031;
- 8lT.-Y. Jian, P.-L. Shao, S. Ye, Chem. Commun. 2011, 47, 2381–2383;
- 8mP. Hu, J. Hu, J. Jiao, X. Tong, Angew. Chem. Int. Ed. 2013, 125, 5427–5430; Angew. Chem. 2013, 52, 5319–5322.
- 9For recent reviews on Brønsted acid catalysis, see:
- 9aH. Yamamoto, J. N. Payette in Hydrogen Bonding in Organic Synthesis (Ed.: ), Wiley-VCH, Weinheim, 2009, pp. 73–140;
10.1002/9783527627844.ch5 Google Scholar
- 9bA. G. Doyle, E. N. Jacobsen, Chem. Rev. 2007, 107, 5713–5743;
- 9cX. Yu, W. Wang, Chem. Asian J. 2008, 3, 516–532;
- 9dD. Kampen, C. M. Reisinger, B. List, Top. Curr. Chem. 2010, 291, 395–456.
- 10For Brønsted acid catalyzed IEDADA reactions, see:
- 10aT. Akiyama, H. Morita, K. Fuchibe, J. Am. Chem. Soc. 2006, 128, 13070–13071;
- 10bH. Liu, G. Dagousset, G. Masson, P. Retailleau, J. Zhu, J. Am. Chem. Soc. 2009, 131, 4598–4599;
- 10cC. Wang, Z.-Y. Han, H.-W. Luo, L.-Z. Gong, Org. Lett. 2010, 12, 2266–2269;
- 10dG. Bergonzini, L. Gramigna, A. Mazzanti, M. Fochi, L. Bernardi, A. Ricci, Chem. Commun. 2010, 46, 327–329;
- 10eH. Xu, S. J. Zuend, M. G. Woll, Y. Tao, E. N. Jacobsen, Science 2010, 327, 986–990;
- 10fG. Dagousset, J. Zhu, G. Masson, J. Am. Chem. Soc. 2011, 133, 14804–14813;
- 10gG. Dagousset, P. Retailleau, G. Masson, J. Zhu, Chem. Eur. J. 2012, 18, 5869–5873;
- 10hL. He, M. Bekkaye, P. Retailleau, G. Masson, Org. Lett. 2012, 14, 3158–3161;
- 10iF. Shi, G.-J. Xing, Z.-L. Tao, S.-W. Luo, S.-J. Tu, L.-Z. Gong, J. Org. Chem. 2012, 77, 6970–6979;
- 10jF. Shi, G.-J. Xing, R.-Y. Zhu, W. Tan, S. Tu, Org. Lett. 2013, 15, 128–131.
- 11For recent reviews on chiral phosphoric acid catalysis, see:
- 11aT. Akiyama, J. Itoh, K. Fuchibe, Adv. Synth. Catal. 2006, 348, 999–1010;
- 11bT. Akiyama, Chem. Rev. 2007, 107, 5744–5758;
- 11cM. Terada, Chem. Commun. 2008, 4097–4112;
- 11dM. Terada, Synthesis 2010, 1929–1982;
- 11eM. Terada, Bull. Chem. Soc. Jpn. 2010, 83, 101–119;
- 11fM. Terada, Curr. Org. Chem. 2011, 15, 2227–2256;
- 11gM. Rueping, A. Kuenkel, I. Atodiresei, Chem. Soc. Rev. 2011, 40, 4539–4549.
- 12Selected reviews dealing with enamides as nucleophiles, see:
- 12aG. Evano, N. Blanchard, M. Toumi, Chem. Rev. 2008, 108, 3054–3131;
- 12bR. Matsubara, S. Kobayashi, Acc. Chem. Res. 2008, 41, 292–301;
- 12cD. R. Carbery, Org. Biomol. Chem. 2008, 6, 3455–3460;
- 12dT. C. Nugent, M. El-Shazly, Adv. Synth. Catal. 2010, 352, 753–819;
- 12eK. Gopalaiah, H. B. Kagan, Chem. Rev. 2011, 111, 4599–4657;
- 12fJ.-H. Xie, S.-F. Zhu, Q.-L. Zhou, Chem. Rev. 2011, 111, 1713–1760.
- 13Selected example of using enamides as nucleophiles in phosphoric acid catalyzed transformations:
- 13aM. Terada, K. Machioka, K. Sorimachi, Angew. Chem. 2006, 118, 2312–2315; Angew. Chem. Int. Ed. 2006, 45, 2254–2257;
- 13bK. Terada, K. Machioka, K. Sorimachi, J. Am. Chem. Soc. 2007, 129, 10336–10337;
- 13cM. Terada, K. Soga, N. Momiyama, Angew. Chem. 2008, 120, 4190–4193; Angew. Chem. Int. Ed. 2008, 47, 4122–4125;
- 13dM. Terada, K. Machioka, K. Sorimachi, Angew. Chem. 2009, 121, 2591–2594; Angew. Chem. Int. Ed. 2009, 48, 2553–2556;
- 13eQ.-X. Guo, Y.-G. Peng, J.-W. Zhang, L. Song, Z. Feng, L.-Z. Gong, Org. Lett. 2009, 11, 4620–4623;
- 13fG. Dagousset, F. Drouet, G. Masson, J. Zhu, Org. Lett. 2009, 11, 5546–5549;
- 13gM. Lu, Y. Lu, D. Zhu, X. Zeng, X. Li, G. Zhong, Angew. Chem. 2010, 122, 8770–8774; Angew. Chem. Int. Ed. 2010, 49, 8588–8592;
- 13hF. Drouet, C. Lalli, H. Liu, G. Masson, J. Zhu, Org. Lett. 2011, 13, 94–97;
- 13iA. Alix, C. Lalli, P. Retailleau, G. Masson, J. Am. Chem. Soc. 2012, 134, 10389–10392.
- 14J. Vicario, D. Aparicio, F. Palacios, Tetrahedron Lett. 2011, 52, 4109–4111.
- 151-Azadienes were used as a syn/anti mixture of isomers: F. Palacios, J. Vicario, D. Aparicio, J. Org. Chem. 2006, 71, 7690–7696.
- 16
- 16aJ. Gonzalez, K. N. Houk, J. Org. Chem. 1992, 57, 3031–3037;
- 16bL. R. Domingo, Tetrahedron 2002, 58, 3765–3774;
- 16cR. G. Parr, L. von Szentpaly, S. Liu, J. Am. Chem. Soc. 1999, 121, 1922–1924.
- 17For stepwise mechanisms for polar Diels–Alder reactions, see: L. R. Domingo, J. A. Sáez, Org. Biomol. Chem. 2009, 7, 3576–3583.
- 18The majority of the unreacted 1-azadienes was recovered.