Bacterial Methanogenesis Proceeds by a Radical Mechanism†
This work was supported by funds from the Deutsche Forschungsgemeinschaft and Synmikro Marburg. I thank Prof. Dr. R. K. Thauer for helpful advice.
Graphical Abstract
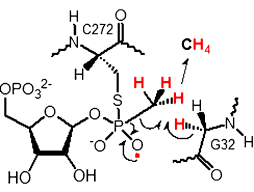
The thiyl radical of cysteine 272 (C272) in the C-P lyase adds to 5-phosphoribose-1-methylphosphonate to give a covalently bound thiophosphonate radical. Reaction with glycine 32 (G32) of the enzyme yields methane, a glycyl radical, and thiophosphate (see scheme). Intramolecular attack of the 2-OH group leads to 5-phosphoribose-1,2-cyclic-phosphate, whereas the glycyl radical oxidizes the liberated SH group back to the thiyl radical.