Generation of Organolithium Compounds bearing Super Silyl Ester and their Application to Matteson Rearrangement†
Susumu Oda
Department of Chemistry, The University of Chicago, 5735 South Ellis Avenue, Chicago, IL 60637 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Hisashi Yamamoto
Department of Chemistry, The University of Chicago, 5735 South Ellis Avenue, Chicago, IL 60637 (USA)
Molecular Catalyst, Research Center, Chubu University, 1200 Matsumoto, Kasugai, Aichi 487-8501 (Japan)
Department of Chemistry, The University of Chicago, 5735 South Ellis Avenue, Chicago, IL 60637 (USA)Search for more papers by this authorSusumu Oda
Department of Chemistry, The University of Chicago, 5735 South Ellis Avenue, Chicago, IL 60637 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Hisashi Yamamoto
Department of Chemistry, The University of Chicago, 5735 South Ellis Avenue, Chicago, IL 60637 (USA)
Molecular Catalyst, Research Center, Chubu University, 1200 Matsumoto, Kasugai, Aichi 487-8501 (Japan)
Department of Chemistry, The University of Chicago, 5735 South Ellis Avenue, Chicago, IL 60637 (USA)Search for more papers by this authorThis work was supported by the NIH (P50GM086145-01). We would like to thank Dr. Antoni Jurkiewicz and Dr. Jin Qin for their expertise in NMR spectroscopy and mass spectrometry, respectively.
Graphical Abstract
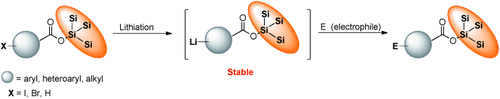
It's super-silyl-fragilithyl-ester-aryl-docious: The super silyl group is a strong protecting group for carboxylic acids and provides a method for direct lithiation that is compatible with the ester moiety. Organolithium compounds bearing a super silyl ester react with a variety of electrophiles in high yields (see scheme). The reaction of lithiated super silyl chloroacetate with a boron compound gives α-functionalization of the ester moiety by Matteson rearrangement.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201304225_sm_miscellaneous_information.pdf15.9 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aV. Snieckus, Chem. Rev. 1990, 90, 879–933;
- 1bM. Schlosser, Eur. J. Org. Chem. 2001, 3975–3984;
- 1cM. Schlosser, Angew. Chem. 2005, 117, 380–398;
10.1002/ange.200300645 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 376–393;
- 1dM. C. Whisler, S. MacNeil, V. Snieckus, P. Beak, Angew. Chem. 2004, 116, 2256–2276;
10.1002/ange.200300590 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 2206–2225.
- 2
- 2aA. Nagaki, H. Kim, Y. Moriwaki, C. Matsuo, J. Yoshida, Chem. Eur. J. 2010, 16, 11167–11177;
- 2bA. Nagaki, H. Kim, J. Yoshida, Angew. Chem. 2008, 120, 7951–7954;
10.1002/ange.200803205 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 7833–7836.
- 3
- 3aX.-J. Wang, X. Sun, L. Zhang, Y. Xu, D. Krishnamurthy, C. H. Senanayake, Org. Lett. 2006, 8, 305–307;
- 3bY. Kondo, N. Takazawa, C. Yamazaki, T. Sakamoto, J. Org. Chem. 1994, 59, 4717–4718.
- 4
- 4aA. Krasovskiy, P. Knochel, Angew. Chem. 2004, 116, 3396–3399;
10.1002/ange.200454084 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 3333–3336;
- 4bL. Boymond, M. Rottländer, G. Chiez, P. Knochel, Angew. Chem. 1998, 110, 1801–1803;
10.1002/(SICI)1521-3757(19980619)110:12<1801::AID-ANGE1801>3.0.CO;2-R Google ScholarAngew. Chem. Int. Ed. 1998, 37, 1701–1703;10.1002/(SICI)1521-3773(19980703)37:12<1701::AID-ANIE1701>3.0.CO;2-U CAS PubMed Web of Science® Google Scholar
- 4cA. Krasovskiy, V. Krasovskaya, P. Knochel, Angew. Chem. 2006, 118, 3024–3027;
10.1002/ange.200504024 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 2958–2961.
- 5
- 5aW. E. Parham, L. D. Jones, J. Org. Chem. 1976, 41, 2704–2706;
- 5bW. E. Parham, Y. A. Syed, J. Org. Chem. 1974, 39, 2053–2056;
- 5cW. E. Parham, C. K. Bradsher, Acc. Chem. Res. 1982, 15, 300–305.
- 6
- 6aM. B. Boxer, H. Yamamoto, J. Am. Chem. Soc. 2006, 128, 48–49;
- 6bM. B. Boxer, H. Yamamoto, Nat. Protoc. 2006, 1, 2434–2438;
- 6cM. B. Boxer, H. Yamamoto, J. Am. Chem. Soc. 2007, 129, 2762–2763;
- 6dM. B. Boxer, H. Yamamoto, Org. Lett. 2008, 10, 453–455;
- 6eM. B. Boxer, M. Akakura, H. Yamamoto, J. Am. Chem. Soc. 2008, 130, 1580–1582;
- 6fM. B. Boxer, B. J. Albert, H. Yamamoto, Aldrichimica Acta 2009, 42, 3–15;
- 6gB. J. Albert, H. Yamamoto, Angew. Chem. 2010, 122, 2807–2809;
10.1002/ange.200907076 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 2747–2749;
- 6hY. Yamaoka, H. Yamamoto, J. Am. Chem. Soc. 2010, 132, 5354–5356;
- 6iB. J. Albert, Y. Yamaoka, H. Yamamoto, Angew. Chem. 2011, 123, 2658–2660; Angew. Chem. Int. Ed. 2011, 50, 2610–2612;
- 6jJ. Saadi, M. Akakura, H. Yamamoto, J. Am. Chem. Soc. 2011, 133, 14248–14251;
- 6kP. B. Brady, H. Yamamoto, Angew. Chem. 2012, 124, 1978–1982;
10.1002/ange.201108325 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 1942–1946.
- 7J. Tan, M. Akakura, H. Yamamoto, Angew. Chem. 2013, DOI: ; Angew. Chem. Int. Ed. 2013, DOI: .
- 8
- 8aJ. Clayden, Organolithiums: Selectivity for Synthesis, Tetrahedron Organic Chemistry Series, Vol. 23;
- 8bM. Yus, R. Ortiz, F. F. Huerta, Tetrahedron 2003, 59, 8525–8542.
- 9The stability of super silyl para-lithiobenzoate was investigated. See the Supporting Information for details.
- 10
- 10aM. A. J. Miah, V. Snieckus, J. Org. Chem. 1985, 50, 5436–5438;
- 10bR. J. Mills, V. Snieckus, J. Org. Chem. 1989, 54, 4386–4390;
- 10cM. Alessi, A. L. Larkin, K. A. Ogilvie, L. A. Green, S. Lai, S. Lopez, V. Snieckus, J. Org. Chem. 2007, 72, 1588–1594.
- 11
- 11aD. S. Matteson, D. J. Majumder, J. Am. Chem. Soc. 1980, 102, 7588–7590;
- 11bD. S. Matteson, D. Ray, J. Am. Chem. Soc. 1980, 102, 7590–7591;
- 11cD. S. Matteson, K. M. Sadhu, J. Am. Chem. Soc. 1983, 105, 2077–2078;
- 11dD. S. Matteson, D. Majumdar, Organometallics 1983, 2, 1529–1535;
- 11eH. C. Brown, M. V. Rangaishenvi, S. Jayaraman, Organometallics 1992, 11, 1948–1954;
- 11fD. S. Matteson, Chem. Rev. 1989, 89, 1535–1551.
- 12
- 12aH. C. Brown, M. M. Rogic, M. W. Rathke, G. W. Kabalka, J. Am. Chem. Soc. 1968, 90, 818–820;
- 12bH. C. Brown, M. M. Rogic, M. W. Rathke, J. Am. Chem. Soc. 1968, 90, 6218–6219;
- 12cH. C. Brown, H. Nambu, M. M. Rogic, J. Am. Chem. Soc. 1969, 91, 6852–6854;
- 12dH. C. Brown, H. Nambu, M. M. Rogic, J. Am. Chem. Soc. 1969, 91, 6854–6855.
- 13
- 13aM. Durandetti, J. Y. Nédélec, J. Périchon, J. Org. Chem. 1996, 61, 1748–1755;
- 13bM. Durandetti, C. Gosmini, J. Périchon, Tetrahedron 2007, 63, 1146–1153;
- 13cW. A. Moradi, S. L. Buchwald, J. Am. Chem. Soc. 2001, 123, 7996–8002;
- 13dN. A. Beare, J. F. Hartwig, J. Org. Chem. 2002, 67, 541–555;
- 13eT. Hama, X. Liu, D. A. Culkin, J. F. Hartwig, J. Am. Chem. Soc. 2003, 125, 11176–11177;
- 13fX. Liu, J. F. Hartwig, J. Am. Chem. Soc. 2004, 126, 5182–5191.
- 14
- 14aJ. L. Stymiest, V. Bagutski, R. French, V. K. Aggarwal, Nature 2008, 456, 778–782;
- 14bJ. L. Stymiest, G. Dutheuil, A. Mahmood, V. K. Aggarwal, Angew. Chem. 2007, 119, 7635–7638;
10.1002/ange.200702146 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 7491–7494;
- 14cV. Bagutski, R. M. French, V. K. Aggarwal, Angew. Chem. 2010, 122, 5268–5271;
10.1002/ange.201001371 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 5142–5145;
- 14dS. P. Thomas, R. M. French, V. Jheengut, V. K. Aggarwal, Chem. Rec. 2009, 9, 24–39;
- 14eR. Robiette, G. U. Fang, J. N. Harvey, V. K. Aggarwal, Chem. Commun. 2006, 741–743;
- 14fG. Y. Fang, O. A. Wallner, N. D. Blasio, X. Ginesta, J. N. Harvey, V. K. Aggarwal, J. Am. Chem. Soc. 2007, 129, 14632–14639.
- 15The use of more stable boronic esters, such as neopentyl glycol and pinacol gave the product in low yields, presumably due to their steric hindrance.
- 16The use of KHMDS for α-alkylation gave comparable results with LiHMDS.