Ligand-Directed Selective Protein Modification Based on Local Single-Electron-Transfer Catalysis†
Dr. Shinichi Sato
Department of Chemistry, Faculty of Science, Gakushuin University, Mejiro, Tokyo 171-8588 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Hiroyuki Nakamura
Department of Chemistry, Faculty of Science, Gakushuin University, Mejiro, Tokyo 171-8588 (Japan)
Department of Chemistry, Faculty of Science, Gakushuin University, Mejiro, Tokyo 171-8588 (Japan)Search for more papers by this authorDr. Shinichi Sato
Department of Chemistry, Faculty of Science, Gakushuin University, Mejiro, Tokyo 171-8588 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Hiroyuki Nakamura
Department of Chemistry, Faculty of Science, Gakushuin University, Mejiro, Tokyo 171-8588 (Japan)
Department of Chemistry, Faculty of Science, Gakushuin University, Mejiro, Tokyo 171-8588 (Japan)Search for more papers by this authorThis work was partially supported by a Grant-in-Aid for Scientific Research on Innovative Areas “Chemical Biology of Natural Products” from The Ministry of Education, Culture, Sports, Science and Technology (Japan).
Graphical Abstract
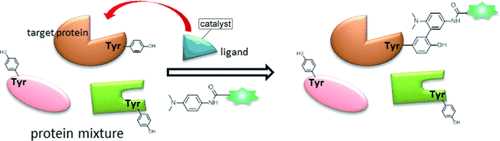
A photocatalyst ([Ru(bpy)3]2+) bound to a protein ligand was essential for the title method. Local single-electron transfer from the catalyst resulted in the formation of tyrosyl radicals. N′-Acetyl-N,N-dimethyl-1,4-phenylenediamine was used as the tyrosyl radical trapping agent and used in a radical addition to afford selective modification of the target protein.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201303831_sm_miscellaneous_information.pdf4.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1T. Hayashi, I. Hamachi, Acc. Chem. Res. 2012, 45, 1460–1469.
- 2A. M. Wu, P. D. Senter, Nat. Biotechnol. 2005, 23, 1137–1146.
- 3J. M. McFarland, M. B. Francis, J. Am. Chem. Soc. 2005, 127, 13490–13491.
- 4G. J. L. Bernardes, J. M. Chalker, J. C. Errey, B. G. Davis, J. Am. Chem. Soc. 2008, 130, 5052–5053.
- 5A. Dondoni, Angew. Chem. 2008, 120, 9133–9135;
10.1002/ange.200802516 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 8995–8997.
- 6J. M. Antos, M. B. Francis, J. Am. Chem. Soc. 2004, 126, 10256–10257.
- 7S. D. Tilley, M. B. Francis, J. Am. Chem. Soc. 2006, 128, 1080–1081.
- 8J. M. Antos, J. M. McFarland, A. T. Iavarone, M. B. Francis, J. Am. Chem. Soc. 2009, 131, 6301–6308.
- 9N. S. Joshi, L. R. Whitaker, M. B. Francis, J. Am. Chem. Soc. 2004, 126, 15942–15943.
- 10H. Ban, J. Gavrilyuk, C. F. Barbas, J. Am. Chem. Soc. 2010, 132, 1523–1525.
- 11K. L. Seim, A. C. Obermeyer, M. B. Francis, J. Am. Chem. Soc. 2011, 133, 16970–16976.
- 12J. Gavrilyuk, H. Ban, M. Nagano, W. Hakamata, C. F. Barbas, Bioconjugate Chem. 2012, 23, 2321–2328.
- 13B. V. Popp, Z. T. Ball, J. Am. Chem. Soc. 2010, 132, 6660–6662.
- 14Z. Chen, B. V. Popp, C. L. Bovet, Z. T. Ball, ACS Chem. Biol. 2011, 6, 920–925.
- 15S. Tsukiji, M. Miyagawa, Y. Takaoka, T. Tamura, I. Hamachi, Nat. Chem. Biol. 2009, 5, 341–343.
- 16S.-h. Fujishima, R. Yasui, T. Miki, A. Ojida, I. Hamachi, J. Am. Chem. Soc. 2012, 134, 3961–3964.
- 17Y. Koshi, E. Nakata, M. Miyagawa, S. Tsukiji, T. Ogawa, I. Hamachi, J. Am. Chem. Soc. 2008, 130, 245–251.
- 18H. Wang, Y. Koshi, D. Minato, H. Nonaka, S. Kiyonaka, Y. Mori, S. Tsukiji, I. Hamachi, J. Am. Chem. Soc. 2011, 133, 12220–12228.
- 19N. Hoffmann, Chem. Rev. 2008, 108, 1052–1103.
- 20K. Zeitler, Angew. Chem. 2009, 121, 9969–9974;
10.1002/ange.200904056 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 9785–9789.
- 21D. A. Fancy, T. Kodadek, Proc. Natl. Acad. Sci. USA 1999, 96, 6020–6024.
- 22K. Kim, D. A. Fancy, D. Carney, T. Kodadek, J. Am. Chem. Soc. 1999, 121, 11896–11897.
- 23The modifications induced by APS were observed in 10 % conversion yield without irradiation with light and/or [Ru(bpy)3]Cl2 in entries 5 and 6, respectively.
- 24R. T. Dean, S. Fu, R. Stocker, M. J. Davies, Biochem. J. 1997, 324 (Pt 1), 1–18.
- 25M. J. Davies, Biochim. Biophys. Acta Proteins Proteomics 2005, 1703, 93–109.
- 26M. J. Davies, Biochem. Biophys. Res. Commun. 2003, 305, 761–770.
- 27V. M. Krishnamurthy, G. K. Kaufman, A. R. Urbach, I. Gitlin, K. L. Gudiksen, D. B. Weibel, G. M. Whitesides, Chem. Rev. 2008, 108, 946–1051.
- 28The use of lower amounts of compound 3 resulted in lower labeling efficiency owing to the tight binding of compound 3 to CA.
- 29We examined the CA inhibitory activity of compounds 3, 4 and [Ru(bpy)3]Cl2 according to the literature protocol reported by J. A. Verpoorte, S. Mehta, J. T. Edsall, J. Biol. Chem. 1967, 242, 4221–4229. Compound 3 showed higher CA inhibitory activity (IC50: 0.50 μM) than compound 4 (IC50: 1.6 μM). [Ru(bpy)3]Cl2 did not show a significant inhibition at 10 μM. See Figure S9 in the Supporting Information.
- 30We examined the selective modification of CA in mouse erythrocyte lysates at various concentrations of LSC 3 and found that the selectivity of the modification at 1 μM was higher than that at 10 μm, although the modification efficiency dropped. Therefore, we carried out the further CA-selective modification experiments in mouse erythrocyte lysates at 1 μM of 3.
- 31Q. Shao, B. Xing, Chem. Commun. 2012, 48, 1739–1741.