Enantioselective CH Arylation Strategy for Functionalized Dibenzazepinones with Quaternary Stereocenters†
Tanguy Saget
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, 1015 Lausanne (Switzerland) http://isic.epfl.ch/lcsa
Search for more papers by this authorCorresponding Author
Prof. Dr. Nicolai Cramer
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, 1015 Lausanne (Switzerland) http://isic.epfl.ch/lcsa
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, 1015 Lausanne (Switzerland) http://isic.epfl.ch/lcsaSearch for more papers by this authorTanguy Saget
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, 1015 Lausanne (Switzerland) http://isic.epfl.ch/lcsa
Search for more papers by this authorCorresponding Author
Prof. Dr. Nicolai Cramer
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, 1015 Lausanne (Switzerland) http://isic.epfl.ch/lcsa
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, 1015 Lausanne (Switzerland) http://isic.epfl.ch/lcsaSearch for more papers by this authorThis work is supported by the European Research Council under the European Community’s Seventh Framework Program (FP7 2007–2013)/ERC Grant agreement no 257891. We thank Dr. R. Scopelliti for X-ray crystallographic analysis of 2 l.
Graphical Abstract
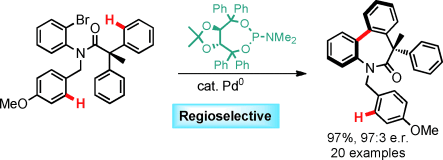
Tada! Highly functionalized chiral dibenzazepinones are obtained by a mild palladium(0)-catalyzed enantioselective CH arylation with excellent selectivities by using simple taddol phosphoramidite ligands. The amide tether allows exclusive regioselectivity through a rare eight-membered palladacycle intermediate.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201303816_sm_miscellaneous_information.pdf6.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews on CH activations, see:
- 1aJ. J. Mousseau, A. B. Charette, Acc. Chem. Res. 2013, 46, 412–424;
- 1bN. Kuhl, M. N. Hopkinson, J. Wencel-Delord, F. Glorius, Angew. Chem. 2012, 124, 10382–10401;
10.1002/ange.201203269 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 10236–10254;
- 1cJ. Yamaguchi, A. D. Yamaguchi, K. Itami, Angew. Chem. 2012, 124, 9092–9142; Angew. Chem. Int. Ed. 2012, 51, 8960–9009;
- 1dK. Hirano, M. Miura, Synlett 2011, 294–307;
- 1eJ. Wencel-Delord, T. Droge, F. Liu, F. Glorius, Chem. Soc. Rev. 2011, 40, 4740–4761;
- 1fL. Ackermann, Chem. Rev. 2011, 111, 1315–1345;
- 1gD. A. Colby, R. G. Bergman, J. A. Ellman, Chem. Rev. 2010, 110, 624–655;
- 1hT. W. Lyons, M. S. Sanford, Chem. Rev. 2010, 110, 1147–1169;
- 1iC.-L. Sun, B.-J. Li, Z.-J. Shi, Chem. Commun. 2010, 46, 677–685;
- 1jR. Jazzar, J. Hitce, A. Renaudat, J. Sofack-Kreutzer, O. Baudoin, Chem. Eur. J. 2010, 16, 2654–2672;
- 1kL. Ackermann, R. Vicente, A. R. Kapdi, Angew. Chem. 2009, 121, 9976–10011; Angew. Chem. Int. Ed. 2009, 48, 9792–9826;
- 1lX Chen, K. M. Engle, D. H. Wang, J. Q. Yu, Angew. Chem. 2009, 121, 5196–5217; Angew. Chem. Int. Ed. 2009, 48, 5094–5115.
- 2R. Giri, B.-F. Shi, K. M. Engle, N. Maugel, J.-Q. Yu, Chem. Soc. Rev. 2009, 38, 3242–3272.
- 3
- 3aD. García-Cuadrado, P. de Mendoza, A. A. C. Braga, F. Maseras, A. M. Echavarren, J. Am. Chem. Soc. 2007, 129, 6880–6886;
- 3bD. Lapointe, K. Fagnou, Chem. Lett. 2010, 39, 1118–1126.
- 4
- 4aM. Lafrance, S. I. Gorelsky, K. Fagnou, J. Am. Chem. Soc. 2007, 129, 14570–14571;
- 4bT. Watanabe, S. Oishi, N. Fujii, H. Ohno, Org. Lett. 2008, 10, 1759–1762;
- 4cM. Chaumontet, R. Piccardi, N. Audic, J. Hitce, J.-L. Peglion, E. Clot, O. Baudoin, J. Am. Chem. Soc. 2008, 130, 15157–15166.
- 5
- 5aM. Lafrance, K. Fagnou, J. Am. Chem. Soc. 2006, 128, 16496–16497;
- 5bM. Livendahl, A. M. Echavarren, Isr. J. Chem. 2010, 50, 630–651.
- 6
- 6aL.-C. Campeau, K. Fagnou, Chem. Commun. 2006, 1253–1264;
- 6bS. Pascual, P. De Mendoza, A. M. Echavarren, Org. Biomol. Chem. 2007, 5, 2727–2734.
- 7
- 7aM. Burwood, B. Davies, I. Diaz, R. Grigg, P. Molina, V. Sridharan, M. Hughes, Tetrahedron Lett. 1995, 36, 9053–9056;
- 7bT. Harayama, H. Akamatsu, K. Okamura, T. Miyagoe, T. Akiyama, H. Abe, Y. Takeuchi, J. Chem. Soc. Perkin Trans. 1 2001, 523–528;
- 7cL. C. Campeau, M. Parisien, M. Leblanc, K. Fagnou, J. Am. Chem. Soc. 2004, 126, 9186–9187;
- 7dM. Leblanc, K. Fagnou, Org. Lett. 2005, 7, 2849–2852;
- 7eL. C. Campeau, M. Parisien, A. Jean, K. Fagnou, J. Am. Chem. Soc. 2006, 128, 581–590;
- 7fC. Huang, V. Gevorgyan, Org. Lett. 2010, 12, 2442–2445;
- 7gD. G. Pintori, M. F. Greaney, J. Am. Chem. Soc. 2011, 133, 1209–1211;
- 7hC. B. Bheeter, J. K. Bera, H. Doucet, Adv. Synth. Catal. 2012, 354, 3533–3538.
- 8For palladium-catalyzed enantioselective CH functionalization to form five-membered rings, see:
- 8aM. Albicker, N. Cramer, Angew. Chem. 2009, 121, 9303–9306;
10.1002/ange.200905060 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 9139–9142;
- 8bM. Nakanishi, D. Katayev, C. Besnard, E. P. Kündig, Angew. Chem. 2011, 123, 7576–7579; Angew. Chem. Int. Ed. 2011, 50, 7438–7441;
- 8cS. Anas, A. Cordi, H. B. Kagan, Chem. Commun. 2011, 47, 11483–11485;
- 8dT. Saget, S. Lemouzy, N. Cramer, Angew. Chem. 2012, 124, 2281–2285;
10.1002/ange.201108511 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 2238–2242;
- 8eD. Katayev, M. Nakanishi, T. Bürgi, E. P. Kündig, Chem. Sci. 2012, 3, 1422–1425;
- 8fN. Martin, C. Pierre, M. Davi, R. Jazzar, O. Baudoin, Chem. Eur. J. 2012, 18, 4480–4484;
- 8gR. Shintani, H. Otomo, K. Ota, T. Hayashi, J. Am. Chem. Soc. 2012, 134, 7305–7308;
- 8hP. A. Donets, T. Saget, N. Cramer, Organometallics 2012, 31, 8040–8046;
- 8iX.-F. Cheng, Y. Li, Y.-M. Su, F. Yin, J.-Y. Wang, J. Sheng, H. U. Vora, X.-S. Wang, J.-Q. Yu, J. Am. Chem. Soc. 2013, 135, 1236–1239.
- 9For palladium-catalyzed enantioselective CH functionalization to form six-membered rings, see: T. Saget, N. Cramer, Angew. Chem. 2012, 124, 13014–13017;
10.1002/ange.201207959 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 12842–12845.
- 10For complementary enantioselective palladium(II)-catalyzed CH functionalizations, see:
- 10aB.-F. Shi, N. Maugel, Y.-H. Zhang, J.-Q. Yu, Angew. Chem. 2008, 120, 4960–4964; Angew. Chem. Int. Ed. 2008, 47, 4882–4886;
- 10bB.-F. Shi, Y.-H. Zhang, J. K. Lam, D.-H. Wang, J.-Q. Yu, J. Am. Chem. Soc. 2010, 132, 460–461;
- 10cM. Wasa, K. M. Engle, D. W. Lin, E. J. Yoo, J.-Q. Yu, J. Am. Chem. Soc. 2011, 133, 19598–19601;
- 10dD.-W. Gao, Y.-C. Shi, Q. Gu, Z.-L. Zhao, S.-L. You, J. Am. Chem. Soc. 2013, 135, 86–89;
- 10eC. Pi, Y. Li, X. Cui, H. Zhang, Y. Han, Y. Wu, Chem. Sci. 2013, 4, 2675–2679.
- 11
- 11aM. Waibel, N. Cramer, Angew. Chem. 2010, 122, 4557–4560;
10.1002/ange.201001752 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 4455–4458;
- 11bM. Pham, B. Ye, N. Cramer, Angew. Chem. 2012, 124, 10762–10766; Angew. Chem. Int. Ed. 2012, 51, 10610–10614;
- 11cB. Ye, N. Cramer, Science 2012, 338, 504–506;
- 11dT. Saget, D. Perez, N. Cramer, Org. Lett. 2013, 15, 1354–1357.
- 12M. Leost, C. Schultz, A. Link, Y.-Z. Wu, J. Biernat, E.-M. Mandelkow, J. A. Bibb, G. L. Snyder, P. Greengard, D. W. Zaharevitz, R. Gussio, A. M. Senderowicz, E. A. Sausville, C. Kunick, L. Meijer, Eur. J. Biochem. 2000, 267, 5983–5994.
- 13
- 13aJ. S. Nair, T. Sheikh, A. L. Ho, G. K. Schwartz, Anticancer Res. 2013, 33, 1307–1316;
- 13bG. T. Wong, D. Manfra, F. M. Poulet, Q. Zhang, H. Josien, T. Bara, L. Engstrom, M. Pinzon-Ortiz, J. S. Fine, H.-J. J. Lee, L. Zhang, G. A. Higgins, E. M. Parker, J. Biol. Chem. 2004, 279, 12876–12882.
- 14I.-M. Shih, T.-L. Wang, Cancer Res. 2007, 67, 1879–1882.
- 15J. L. Jankowsky, D. J. Fadale, J. Anderson, G. M. Xu, V. Gonzales, N. A. Jenkins, N. G. Copeland, M. K. Lee, L. H. Younkin, S. L. Wagner, S. G. Younkin, D. R. Borchelt, Hum. Mol. Genet. 2004, 13, 159–170.
- 16H. W. Lam, Synthesis 2011, 2011–2043.
- 17D. M. Norton, E. A. Mitchell, N. R. Botros, P. G. Jessop, M. C. Baird, J. Org. Chem. 2009, 74, 6674–6680.
- 18An opposite selectivity was observed in Ref. [4a].
- 19Crystallographic data for 2 r: CCDC 936820 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 20H. Tabata, H. Suzuki, K. Akiba, H. Takahashi, H. Natsugari, J. Org. Chem. 2010, 75, 5984–5993.