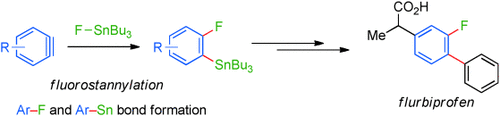
Synchronous ArF and ArSn Bond Formation through Fluorostannylation of Arynes†
Corresponding Author
Prof. Dr. Hiroto Yoshida
Department of Applied Chemistry, Graduate School of Engineering, Hiroshima University, Higashi-Hiroshima 739-8527 (Japan)
Department of Applied Chemistry, Graduate School of Engineering, Hiroshima University, Higashi-Hiroshima 739-8527 (Japan)Search for more papers by this authorRyuma Yoshida
Department of Applied Chemistry, Graduate School of Engineering, Hiroshima University, Higashi-Hiroshima 739-8527 (Japan)
Search for more papers by this authorProf. Dr. Ken Takaki
Department of Applied Chemistry, Graduate School of Engineering, Hiroshima University, Higashi-Hiroshima 739-8527 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Hiroto Yoshida
Department of Applied Chemistry, Graduate School of Engineering, Hiroshima University, Higashi-Hiroshima 739-8527 (Japan)
Department of Applied Chemistry, Graduate School of Engineering, Hiroshima University, Higashi-Hiroshima 739-8527 (Japan)Search for more papers by this authorRyuma Yoshida
Department of Applied Chemistry, Graduate School of Engineering, Hiroshima University, Higashi-Hiroshima 739-8527 (Japan)
Search for more papers by this authorProf. Dr. Ken Takaki
Department of Applied Chemistry, Graduate School of Engineering, Hiroshima University, Higashi-Hiroshima 739-8527 (Japan)
Search for more papers by this authorWe thank Central Glass Co. Ltd. for a generous gift of trifluoromethanesulfonic anhydride.
Graphical Abstract
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201302783_sm_miscellaneous_information.pdf4.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1T. Furuya, J. E. M. N. Klein, T. Ritter, Synthesis 2010, 1804.
- 2
- 2aT. Hiyama Organofluorine Compounds: Chemistry and Applications, Springer, Berlin, 2000;
10.1007/978-3-662-04164-2 Google Scholar
- 2bM. Shimizu, T. Hiyama, Angew. Chem. 2005, 117, 218; Angew. Chem. Int. Ed. 2005, 44, 214;
- 2cK. Müller, C. Faeh, F. Diederich, Science 2007, 317, 1881;
- 2dS. Purser, P. R. Moore, S. Swallow, V. Gouverneur, Chem. Soc. Rev. 2008, 37, 320;
- 2eS. M. Ametamey, M. Honer, P. A. Schubiger, Chem. Rev. 2008, 108, 1501;
- 2fT. Furuya, A. S. Kamlet, T. Ritter, Nature 2011, 473, 470.
- 3G. Balz, G. Schiemann, Ber. Deutsch. Chem. Ges. 1927, 60, 1186.
- 4
- 4aS. D. Kuduk, R. M. DiPardo, M. G. Bock, Org. Lett. 2005, 7, 577;
- 4bH. Sun, S. G. DiMagno, Angew. Chem. 2006, 118, 2786; Angew. Chem. Int. Ed. 2006, 45, 2720;
- 4cH. Sun, S. G. DiMagno, Chem. Commun. 2007, 528.
- 5
- 5aS. Yamada, A. Gavryushin, P. Knochel, Angew. Chem. 2010, 122, 2261; Angew. Chem. Int. Ed. 2010, 49, 2215;
- 5bP. Anbarasan, H. Neumann, M. Beller, Angew. Chem. 2010, 122, 2265;
10.1002/ange.200905855 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 2219;
- 5cP. Anbarasan, H. Neumann, M. Beller, Chem. Asian J. 2010, 5, 1775.
- 6
- 6aK. L. Hull, W. Q. Anani, M. S. Sanford, J. Am. Chem. Soc. 2006, 128, 7134;
- 6bT. Furuya, H. M. Kaiser, T. Ritter, Angew. Chem. 2008, 120, 6082;
10.1002/ange.200802164 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 5993;
- 6cT. Furuya, T. Ritter, J. Am. Chem. Soc. 2008, 130, 10060;
- 6dT. Furuya, A. E. Strom, T. Ritter, J. Am. Chem. Soc. 2009, 131, 1662;
- 6eN. D. Ball, M. S. Sanford, J. Am. Chem. Soc. 2009, 131, 3796;
- 6fX. Wang, T.-S. Mei, J.-Q. Yu, J. Am. Chem. Soc. 2009, 131, 7520;
- 6gT. Furuya, T. Ritter, Org. Lett. 2009, 11, 2860;
- 6hD. A. Watson, M. Su, G. Teverovskiy, Y. Zhang, J. García-Fortanet, T. Kinzel, S. L. Buchwald, Science 2009, 325, 1661;
- 6iT. Furuya, D. Benitez, E. Tkatchouk, A. E. Strom, P. Tang, W. A. Goddard III, T. Ritter, J. Am. Chem. Soc. 2010, 132, 3793;
- 6jP. Tang, T. Furuya, T. Ritter, J. Am. Chem. Soc. 2010, 132, 12150;
- 6kP. Tang, T. Ritter, Tetrahedron 2011, 67, 4449.
- 7
- 7aH. Hayashi, H. Sonoda, K. Fukumura, T. Nagata, Chem. Commun. 2002, 1618;
- 7bP. Tang, W. Wang, T. Ritter, J. Am. Chem. Soc. 2011, 133, 11482.
- 8
- 8aM. S. Butler, M. A. Cooper, J. Antibiot. 2011, 64, 413;
- 8bM. V. Stundick, M. Metz, A. Sampath, J. C. Larsen, Drug Dev. Res. 2011, 72, 361;
- 8cK. Bush, M. J. Pucci, Biochem. Pharmacol. 2011, 82, 1528.
- 9For reviews, see:
- 9aD. Peña, D. Pérez, E. Gutián, Angew. Chem. 2006, 118, 3659;
10.1002/ange.200600291 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 3579;
- 9bH. Yoshida, J. Ohshita, A. Kunai, Bull. Chem. Soc. Jpn. 2010, 83, 199.
- 10
- 10aH. Yoshida, E. Shirakawa, Y. Honda, T. Hiyama, Angew. Chem. 2002, 114, 3381;
Angew. Chem. Int. Ed. 2002, 41, 3247;
10.1002/1521-3773(20020902)41:17<3247::AID-ANIE3247>3.0.CO;2-P CAS PubMed Web of Science® Google Scholar
- 10bH. Yoshida, T. Terayama, J. Ohshita, A. Kunai, Chem. Commun. 2004, 1980;
- 10cU. K. Tambar, B. M. Stoltz, J. Am. Chem. Soc. 2005, 127, 5340;
- 10dH. Yoshida, M. Watanabe, J. Ohshita, A. Kunai, Chem. Commun. 2005, 3292;
- 10eH. Yoshida, T. Minabe, J. Ohshita, A. Kunai, Chem. Commun. 2005, 3454;
- 10fH. Yoshida, M. Watanabe, J. Ohshita, A. Kunai, Tetrahedron Lett. 2005, 46, 6729;
- 10gH. Yoshida, M. Watanabe, J. Ohshita, A. Kunai, Chem. Lett. 2005, 34, 1538;
- 10hZ. Liu, R. Larock, J. Am. Chem. Soc. 2005, 127, 13112;
- 10iH. Yoshida, M. Watanabe, T. Morishita, J. Ohshita, A. Kunai, Chem. Commun. 2007, 1505;
- 10jH. Yoshida, Y. Mimura, J. Ohshita, A. Kunai, Chem. Commun. 2007, 2405;
- 10kS. Beltrán-Rodil, D. Peña, E. Guitián, Synlett 2007, 1308;
- 10lH. Yoshida, T. Kishida, M. Watanabe, J. Ohshita, Chem. Commun. 2008, 5963;
- 10mD. G. Pintori, M. F. Greaney, Org. Lett. 2010, 12, 168;
- 10nH. Yoshida, Y. Ito, Y. Yoshikawa, J. Ohshita, K. Takaki, Chem. Commun. 2011, 47, 8664.
- 11Arynes are well-known as soft electrophiles. See: S. V. Kessar in Comprehensive Organic Synthesis Vol. 4 (Eds.: ), Pergamon, Oxford, 1991, p. 483.
10.1016/B978-0-08-052349-1.00101-3 Google Scholar
- 12Although we have also examined an insertion reaction of an aryne into a fluorine–carbonyl σ-bond of an acid fluoride, the desired product was not formed at all in contrast to the results with an acid chloride and bromide. See Ref. [10j].
- 13For insertion reactions of arynes into Sn-containing σ bonds, see:
- 13aH. Yoshida, K. Tanino, J. Ohshita, A. Kunai, Angew. Chem. 2004, 116, 5162; Angew. Chem. Int. Ed. 2004, 43, 5052;
- 13bH. Yoshida, K. Tanino, J. Ohshita, A. Kunai, Chem. Commun. 2005, 5678;
- 13cB. V. Lakshmi, U. K. Wefelscheid, U. Kazmaier, Synlett 2011, 345. See also ref. [10b].
- 14
- 14aY. Himeshima, T. Sonoda, H. Kobayashi, Chem. Lett. 1983, 1211;
- 14bD. Peña, A. Cobas, D. Pérez, E. Gutián, Synthesis 2002, 1454.
- 15Nucleophilic attack to 3-methoxybenzyne always takes place at the meta position to the methoxy group; this can rationally be explained by the inductive electron-withdrawing effect of the methoxy group as well as the steric repulsion. Similarly, 3-bromobenzyne and 3-chlorobenzyne would accept nucleophiles at the meta position to the halogen moiety owing to its strong electron-withdrawing effect. For reviews on arynes, see: Refs. [9b] and [11].
- 16C. D. Campbell, C. W. Rees, J. Chem. Soc. C 1969, 742.
- 17Bu3SnF has proven to be insoluble in DME. See the Supporting Information for details. For insoluble nature of Bu3SnF, see:
- 17aT. N. Mitchell, K. Kwetkat, B. Godry, Organometallics 1991, 10, 1633;
- 17bP. D. Lickiss, R. Lucas, J. Inorg. Organomet. Polym. 1995, 5, 247.
- 18Triorganotin fluorides are apt to form difluorotriorganostannates by action with a fluoride ion, see:
- 18aS. L. Blunden, R. Hill, J. Organomet. Chem. 1989, 371, 145;
- 18bR. Bujok, M. Makosza, Synlett 2004, 371;
- 18cM. Makosza, R. Bujok, J. Fluorine Chem. 2005, 126, 209.
- 19Monitoring the reaction by 19F NMR spectroscopy has shown that the fluorostannylation product exists not as 6 but as 2, even before work-up.
- 20There have been limited examples of the reaction of arynes with a fluoride ion. See:
- 20aA. A. Kolomeitsev, M. Vorobyev, H. Gillandt, Tetrahedron Lett. 2008, 49, 449;
- 20bV. V. Grushin, W. J. Marshall, Organometallics 2008, 27, 4825;
- 20cV. Diemer, J. S. Garcia, F. R. Leroux, F. Colobert, J. Fluorine Chem. 2012, 134, 146.
- 21In addition to this result, the reaction of 1 a, a fluoride ion, and 4-(trifluoromethyl)benzaldehyde did not give 2-fluoro-4′-(trifluoromethyl)benzhydrol at all, thus also suggesting the absence of 2-fluoroaryl anion 4. See: H. Yoshida, M. Watanabe, H. Fukushima, J. Ohshita, A. Kunai, Org. Lett. 2004, 6, 4049.
- 22For similar electron-donating effect of a trimethylsilyl moiety in the reaction of 3-(trimethylsilyl)benzyne, see: T. Ikawa, T. Nishiyama, T. Shigeta, S. Mohri, S. Morita, S. Takayanagi, Y. Terauchi, Y. Morikawa, A. Takagi, Y. Ishikawa, S. Fujii, Y. Kita, S. Akai, Angew. Chem. 2011, 123, 5792;
10.1002/ange.201100360 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 5674.
- 23For the aryne distortion model for understanding regioselectivity, see:
- 23aP. H.-Y. Cheong, R. S. Paton, S. M. Bronner, G-Y. J. Im, N. K. Garg, K. N. Houk, J. Am. Chem. Soc. 2010, 132, 1267;
- 23bG-Y. J. Im, S. M. Bronner, A. E. Goetz, R. S. Paton, P. H.-Y. Cheong, K. N. Houk, N. K. Garg, J. Am. Chem. Soc. 2010, 132, 17933;
- 23cS. M. Bronner, J. L. Mackey, K. N. Houk, N. K. Garg, J. Am. Chem. Soc. 2012, 134, 13966.
- 24
- 24aS. Takahashi, M. Uno, K. Seto, S. Mototani (Nitto Chemical Industry Co., Ltd.), Jpn. Kokai Tokkyo Koho JP 62019551, 1987;
- 24bK. Kobayashi, Y. Yamamoto, N. Miyaura, Organometallics 2011, 30, 6323.