Synthesis of a Silylium Zwitterion†
Rodrigo Ramírez-Contreras
Department of Chemistry, Texas A& M University, TAMU-3255, College Station, TX 77842 (USA) http://www.chem.tamu.edu/rgroup/ozerov/
Search for more papers by this authorDr. Nattamai Bhuvanesh
Department of Chemistry, Texas A& M University, TAMU-3255, College Station, TX 77842 (USA) http://www.chem.tamu.edu/rgroup/ozerov/
Search for more papers by this authorDr. Jia Zhou
Department of Chemistry, Texas A& M University, TAMU-3255, College Station, TX 77842 (USA) http://www.chem.tamu.edu/rgroup/ozerov/
Search for more papers by this authorCorresponding Author
Prof. Oleg V. Ozerov
Department of Chemistry, Texas A& M University, TAMU-3255, College Station, TX 77842 (USA) http://www.chem.tamu.edu/rgroup/ozerov/
Department of Chemistry, Texas A& M University, TAMU-3255, College Station, TX 77842 (USA) http://www.chem.tamu.edu/rgroup/ozerov/Search for more papers by this authorRodrigo Ramírez-Contreras
Department of Chemistry, Texas A& M University, TAMU-3255, College Station, TX 77842 (USA) http://www.chem.tamu.edu/rgroup/ozerov/
Search for more papers by this authorDr. Nattamai Bhuvanesh
Department of Chemistry, Texas A& M University, TAMU-3255, College Station, TX 77842 (USA) http://www.chem.tamu.edu/rgroup/ozerov/
Search for more papers by this authorDr. Jia Zhou
Department of Chemistry, Texas A& M University, TAMU-3255, College Station, TX 77842 (USA) http://www.chem.tamu.edu/rgroup/ozerov/
Search for more papers by this authorCorresponding Author
Prof. Oleg V. Ozerov
Department of Chemistry, Texas A& M University, TAMU-3255, College Station, TX 77842 (USA) http://www.chem.tamu.edu/rgroup/ozerov/
Department of Chemistry, Texas A& M University, TAMU-3255, College Station, TX 77842 (USA) http://www.chem.tamu.edu/rgroup/ozerov/Search for more papers by this authorWe are thankful for the support of this research by the Office of Science of the US Department of Energy, Basic Energy Sciences (grant DE-FG02-10ER16201) and the Welch Foundation (grant A-1717).
Graphical Abstract
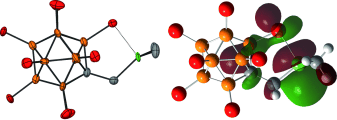
A zwitterionic construction has been synthesized that covalently links a trialkylsilylium cation and a weakly coordinating carborane anion in one neutral molecule (see X-ray structure; Si green, C gray, B orange, Cl red). The silylium character of this molecule is supported by DFT calculations (see LUMO), 29Si CP/MAS NMR spectroscopy, and reactivity.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201302082_sm_miscellaneous_information.pdf1.6 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. B. Lambert, L. Kania, S. Zhang, Chem. Rev. 1995, 95, 1191.
- 2C. A. Reed, Acc. Chem. Res. 1998, 31, 325.
- 3C. A. Reed, Acc. Chem. Res. 2010, 43, 121.
- 4C. A. Reed, Z. Xie, R. Bau, A. Benesi, Science 1993, 262, 402.
- 5J. B. Lambert, S. Zhang, J. Chem. Soc. Chem. Commun. 1993, 383.
- 6J. B. Lambert, S. Zhang, C. L. Stern, J. C. Huffman, Science 1993, 260, 1917.
- 7K.-C. Kim, C. A. Reed, D. W. Elliott, L. J. Mueller, F. Tham, L. Lin, J. B. Lambert, Science 2002, 297, 825.
- 8I. Krossing, I. Raabe, Angew. Chem. 2004, 116, 2116; Angew. Chem. Int. Ed. 2004, 43, 2066.
- 9S. Körbe, P. J. Schreiber, J. Michl, Chem. Rev. 2006, 106, 5208.
- 10E. S. Stoyanov, K.-C. Kim, C. A. Reed, J. Am. Chem. Soc. 2006, 128, 8500.
- 11A. Schäfer, M. Reißmann, A. Schäfer, W. Saak, D. Haase, T. Müller, Angew. Chem. 2011, 123, 12845;
10.1002/ange.201106582 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 12636.
- 12Z. Xie, J. Manning, R. W. Reed, R. Mathur, P. D. W. Boyd, A. Benesi, C. A. Reed, J. Am. Chem. Soc. 1996, 118, 2922.
- 13S. P. Hoffmann, T. Kato, F. S. Tham, C. A. Reed, Chem. Commun. 2006, 767.
- 14M. F. Ibad, P. Langer, A. Schulz, A. Villinger, J. Am. Chem. Soc. 2011, 133, 21016.
- 15H. F. T. Klare, M. Oestreich, Dalton Trans. 2010, 39, 9176.
- 16C. Douvris, O. V. Ozerov, Science 2008, 321, 1188.
- 17O. Allemann, S. Duttwyler, P. Romanato, K. K. Baldridge, J. S. Siegel, Science 2011, 332, 574.
- 18E. S. Stoyanov, S. P. Hoffmann, M. Juhasz, C. A. Reed, J. Am. Chem. Soc. 2006, 128, 3160.
- 19J. D. Egbert, R. M. Bullock, D. M. Heinekey, Organometallics 2007, 26, 2291.
- 20G. Erker, Dalton Trans. 2005, 1883.
- 21W. E. Piers, T. Chivers, Chem. Soc. Rev. 1997, 26, 345.
- 22L. O. Müller, D. Himmel, J. Stauffer, G. Steinfeld, J. Slattery, G. Santiso-Quiñones, V. Brecht, I. Krossing, Angew. Chem. 2008, 120, 7772; Angew. Chem. Int. Ed. 2008, 47, 7659.
- 23A. Kraft, N. Trapp, D. Himmel, H. Böhrer, P. Schlüter, H. Scherer, I. Krossing, Chem. Eur. J. 2012, 18, 9371.
- 24R. Ramírez-Contreras, O. V. Ozerov, Dalton Trans. 2012, 41, 7842.
- 25C. Douvris, C. M. Nagaraja, C.-H. Chen, B. M. Foxman, O. V. Ozerov, J. Am. Chem. Soc. 2010, 132, 4946.
- 26T. Küppers, E. Bernhardt, R. Eujen, H. Willner, C. W. Lehmann, Angew. Chem. 2007, 119, 6462;
10.1002/ange.200701136 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 6346.
- 27T. Kato, C. A. Reed, Angew. Chem. 2004, 116, 2968; Angew. Chem. Int. Ed. 2004, 43, 2908.
- 28F. Scholz, D. Himmel, H. Scherer, I. Krossing, Chem. Eur. J. 2013, 19, 109.
- 29Warming up the SO2 solution of 3 to ambient temperature resulted in decomposition, although the diminished 1H NMR resonance of [(CH3)3C]+ could still be observed at 20 °C. Apparently, strong acid formation takes place, from the similarity of new downfield signals to the spectrum of F3CSO3H in liquid SO2. It is not clear whether degradation is owing to interaction with SO2, adventitious impurities, or the anion. Kato and Reed[27] also only observed [(CH3)3C][HCB11Me5Br6] in solution at −60 °C.
- 30I. Zharov, B. T. King, Z. Havlas, A. Pardi, J. Michl, J. Am. Chem. Soc. 2000, 122, 10253.
- 31M. J. Ingleson, M. F. Mahon, A. S. Weller, Chem. Commun. 2004, 2398.