Noncovalent Interactions in Organocatalysis: Modulating Conformational Diversity and Reactivity in the MacMillan Catalyst†
Mareike C. Holland
Organisch Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster (Germany) http://www.uni-muenster.de/Chemie.oc/gilmour/
Laboratorium für Organische Chemie, ETH Zürich (Switzerland)
Search for more papers by this authorShyeni Paul
Department Chemie, Ludwig-Maximilians-Universität München (Germany)
Search for more papers by this authorDr. W. Bernd Schweizer
Laboratorium für Organische Chemie, ETH Zürich (Switzerland)
Search for more papers by this authorDr. Klaus Bergander
Organisch Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster (Germany) http://www.uni-muenster.de/Chemie.oc/gilmour/
Search for more papers by this authorDr. Christian Mück-Lichtenfeld
Organisch Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster (Germany) http://www.uni-muenster.de/Chemie.oc/gilmour/
Search for more papers by this authorCorresponding Author
Dr. Sami Lakhdar
Laboratoire de Chimie Moléculaire et Thio-organique, ENSICAEN, 6 Boulevard Maréchal Juin, 14050 Caen (France)
Sami Lakhdar, Laboratoire de Chimie Moléculaire et Thio-organique, ENSICAEN, 6 Boulevard Maréchal Juin, 14050 Caen (France)
Ryan Gilmour, Organisch Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster (Germany) http://www.uni-muenster.de/Chemie.oc/gilmour/
Search for more papers by this authorProf. Dr. Herbert Mayr
Department Chemie, Ludwig-Maximilians-Universität München (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Ryan Gilmour
Organisch Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster (Germany) http://www.uni-muenster.de/Chemie.oc/gilmour/
Sami Lakhdar, Laboratoire de Chimie Moléculaire et Thio-organique, ENSICAEN, 6 Boulevard Maréchal Juin, 14050 Caen (France)
Ryan Gilmour, Organisch Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster (Germany) http://www.uni-muenster.de/Chemie.oc/gilmour/
Search for more papers by this authorMareike C. Holland
Organisch Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster (Germany) http://www.uni-muenster.de/Chemie.oc/gilmour/
Laboratorium für Organische Chemie, ETH Zürich (Switzerland)
Search for more papers by this authorShyeni Paul
Department Chemie, Ludwig-Maximilians-Universität München (Germany)
Search for more papers by this authorDr. W. Bernd Schweizer
Laboratorium für Organische Chemie, ETH Zürich (Switzerland)
Search for more papers by this authorDr. Klaus Bergander
Organisch Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster (Germany) http://www.uni-muenster.de/Chemie.oc/gilmour/
Search for more papers by this authorDr. Christian Mück-Lichtenfeld
Organisch Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster (Germany) http://www.uni-muenster.de/Chemie.oc/gilmour/
Search for more papers by this authorCorresponding Author
Dr. Sami Lakhdar
Laboratoire de Chimie Moléculaire et Thio-organique, ENSICAEN, 6 Boulevard Maréchal Juin, 14050 Caen (France)
Sami Lakhdar, Laboratoire de Chimie Moléculaire et Thio-organique, ENSICAEN, 6 Boulevard Maréchal Juin, 14050 Caen (France)
Ryan Gilmour, Organisch Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster (Germany) http://www.uni-muenster.de/Chemie.oc/gilmour/
Search for more papers by this authorProf. Dr. Herbert Mayr
Department Chemie, Ludwig-Maximilians-Universität München (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Ryan Gilmour
Organisch Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster (Germany) http://www.uni-muenster.de/Chemie.oc/gilmour/
Sami Lakhdar, Laboratoire de Chimie Moléculaire et Thio-organique, ENSICAEN, 6 Boulevard Maréchal Juin, 14050 Caen (France)
Ryan Gilmour, Organisch Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster (Germany) http://www.uni-muenster.de/Chemie.oc/gilmour/
Search for more papers by this authorWe acknowledge generous financial support from the WWU Münster, the DFG (SFB 749), and the ETH Zürich (ETH Independent Investigator Research Award to R.G.).
Graphical Abstract
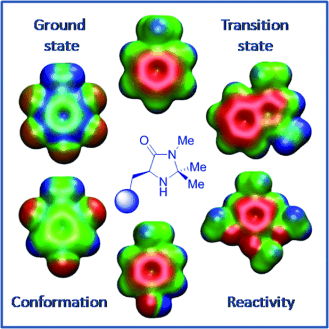
Intriguing imidazolidinones! Inspired by noncovalent interactions in proteins, a series of electronically distinct MacMillan catalysts were designed. The effect of electronic modulation on ground state conformation, reactivity, and performance was studied in a catalytic setting with intriguing outcomes.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201301864_sm_miscellaneous_information.pdf2.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Asymmetric Organocatalysis: From Biomimetic Concepts to Applications in Asymmetric Synthesis (Eds.: ), Wiley-VCH, Weinheim, 2005.
- 2
- 2aJ. R. Knowles, Nature 1991, 350, 121–124;
- 2bR. R. Knowles, E. N. Jacobsen, Proc. Natl. Acad. Sci. USA 2010, 107, 20678–20685.
- 3J.-M. Lehn, Science 1993, 260, 1762–1763.
- 4D. W. C. MacMillan, Nature 2008, 455, 304–308.
- 5K. A. Ahrendt, C. J. Borths, D. W. C. MacMillan, J. Am. Chem. Soc. 2000, 122, 4243–4244.
- 6
- 6aM. Brandl, M. S. Weiss, A. Jabs, J. Sühnel, R. Hilgenfeld, J. Mol. Biol. 2001, 307, 357–377;
- 6bO. Takahashi, Y. Kohono, M. Nishio, Chem. Rev. 2010, 110, 6049–6076.
- 7G. B. McGaughey, M. Gagné, A. K. Rappé, J. Biol. Chem. 1998, 273, 15458–15463.
- 8For the X-ray structure of the MacMillan first-generation catalyst see: J. C. Burley, R. Gilmour, T. J. Prior, G. M. Day, Acta Crystallogr. Sect. C 2008, 64, o 10–o14.
- 9For selected examples see:
- 9aD. Seebach, U. Grošelj, M. D. Badine, W. B. Schweizer, A. K. Beck, Helv. Chim. Acta 2008, 91, 1999–2034;
- 9bU. Grošelj, W. B. Schweizer, M.-O. Ebert, D. Seebach, Helv. Chim. Acta 2009, 92, 1–13;
- 9cJ. B. Brazier, G. Evans, T. J. K. Gibbs, S. J. Coles, M. B. Hursthouse, J. A. Platts, N. C. O. Tomkinson, Org. Lett. 2009, 11, 133–136;
- 9dD. Seebach, R. Gilmour, U. Grošelj, G. Deniau, C. Sparr, M.-O. Ebert, A. K. Beck, L. B. McCusker, D. Sisak, Helv. Chim. Acta 2010, 93, 603–634;
- 9eC. Sparr, R. Gilmour, Angew. Chem. 2010, 122, 6670–6673;
10.1002/ange.201003734 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 6520–6523;
- 9fC. Sparr, R. Gilmour, Angew. Chem. 2011, 123, 8541–8545;
10.1002/ange.201103360 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 8391–8395;
- 9gFor an excellent review of theoretical investigations of organocatalysis see: P. H.-Y. Cheong, C. Y. Legault, J. M. Um, N. Çelebi-Ölçüm, K. N. Houk, Chem. Rev. 2011, 111, 5042–5137, and references therein.
- 10M. Nishio, M. Hirota, Y. Umezawa, The CH/π Interaction: Evidence, Nature and Consequences, Wiley-VCH, Weinheim, 1998.
- 11R. Gordillo, J. Carter, K. N. Houk, Adv. Synth. Catal. 2004, 346, 1175–1185.
- 12For examples from this laboratory see: C. Sparr, W. B. Schweizer, H. M. Senn, R. Gilmour, Angew. Chem. 2009, 121, 3111–3114;
10.1002/ange.200900405 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 3065–3068, and references [9f] and [11].
- 13925388 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 14925387 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 15D. Seebach, U. Grošelj, W. B. Schweizer, S. Grimme, C. Mück-Lichtenfeld, Helv. Chim. Acta 2010, 93, 1–16.
- 16
- 16aC. Altona in Encyclopedia of NMR (Eds.: ), Wiley, New York, 1996;
- 16bE. Díez, J. San-Fabián, J. Guilleme, C. Altona, L. A. Donders, Mol. Phys. 1989, 68, 49–63.
- 17A. Navarro-Vazquez, J. C. Cobas, F. J. Sardina, J. Chem. Inf. Comput. Sci. 2004, 44, 1680–1685.
- 18H. Mayr, T. Bug, M. F. Gotta, N. Hering, B. Irrgang, B. Janker, B. Kempf, R. Loos, A. R. Ofial, G. Remennikov, H. Schimmel, J. Am. Chem. Soc. 2001, 123, 9500–9512.
- 19
- 19aS. Lakhdar, T. Tokuyasu, H. Mayr, Angew. Chem. 2008, 120, 8851–8854;
10.1002/ange.200802889 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 8723–8726;
- 19bS. Lakhdar, J. Ammer, H. Mayr, Angew. Chem. 2011, 123, 10127–10130;
10.1002/ange.201103683 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 9953–9956;
- 19cH. Mayr, S. Lakhdar, B. Maji, A. R. Ofial, Beilstein J. Org. Chem. 2012, 8, 1458–1478.
- 20N. A. Paras, D. W. C. MacMillan, J. Am. Chem. Soc. 2001, 123, 4370–4371.
- 21S. Lakhdar, H. Mayr, Chem. Commun. 2011, 47, 1866–1868.
- 22J. F. Austin, D. W. C. MacMillan, J. Am. Chem. Soc. 2002, 124, 1172–1173.
- 23
- 23aJ. C. Ma, D. A. Dougherty, Chem. Rev. 1997, 97, 1303–1324;
- 23bJ. P. Gallivan, D. A. Dougherty, Proc. Natl. Acad. Sci. USA 1999, 96, 9459–9464;
- 23cL. M. Salonen, M. Ellermann, F. Diederich, Angew. Chem. 2011, 123, 4908–4944;
10.1002/ange.201007560 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 4808–4842.
- 24Y. Mori, S. Yamada, Molecules 2012, 17, 2161–2168.