The Binding of Benzoarylsulfonamide Ligands to Human Carbonic Anhydrase is Insensitive to Formal Fluorination of the Ligand†
Dr. Matthew R. Lockett
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, MA 02138 (USA)
These authors contributed equally to this work.
Search for more papers by this authorDr. Heiko Lange
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, MA 02138 (USA)
These authors contributed equally to this work.
Search for more papers by this authorDr. Benjamin Breiten
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, MA 02138 (USA)
These authors contributed equally to this work.
Search for more papers by this authorDr. Annie Heroux
Photon Sciences Directorate, Building 745, Brookhaven National Laboratory, Upton, NY 11973 (USA)
Search for more papers by this authorDr. Woody Sherman
Schrödinger Inc., 120 West 45 th Street, New York, NY 10036 (USA)
Search for more papers by this authorDr. Dmitrij Rappoport
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, MA 02138 (USA)
Search for more papers by this authorPatricia O. Yau
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, MA 02138 (USA)
Search for more papers by this authorDr. Philip W. Snyder
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, MA 02138 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. George M. Whitesides
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, MA 02138 (USA)
Wyss Institute for Biologically Inspired Engineering, Harvard University, 60 Oxford Street, Cambridge, MA 02138 (USA)
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, MA 02138 (USA)Search for more papers by this authorDr. Matthew R. Lockett
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, MA 02138 (USA)
These authors contributed equally to this work.
Search for more papers by this authorDr. Heiko Lange
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, MA 02138 (USA)
These authors contributed equally to this work.
Search for more papers by this authorDr. Benjamin Breiten
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, MA 02138 (USA)
These authors contributed equally to this work.
Search for more papers by this authorDr. Annie Heroux
Photon Sciences Directorate, Building 745, Brookhaven National Laboratory, Upton, NY 11973 (USA)
Search for more papers by this authorDr. Woody Sherman
Schrödinger Inc., 120 West 45 th Street, New York, NY 10036 (USA)
Search for more papers by this authorDr. Dmitrij Rappoport
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, MA 02138 (USA)
Search for more papers by this authorPatricia O. Yau
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, MA 02138 (USA)
Search for more papers by this authorDr. Philip W. Snyder
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, MA 02138 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. George M. Whitesides
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, MA 02138 (USA)
Wyss Institute for Biologically Inspired Engineering, Harvard University, 60 Oxford Street, Cambridge, MA 02138 (USA)
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, MA 02138 (USA)Search for more papers by this authorThe authors thank Dr. Jasmin Mecinovic, Dr. Ramani Ranatunge, Dr. Demetri Moustakas, Dr. Manza Atkinson, Dr. Mohammad Al-Sayah, Dr. Shuji Fujita, and Mr. Jang Hoon Yoon for their technical contributions. This work was supported by the National Science Foundation (CHE-1152196) and the Wyss Institute of Biologically Inspired Engineering. H.L. thanks the Deutsche Forschungsgemeinschaft (DFG) for a postdoctoral stipend.
Graphical Abstract
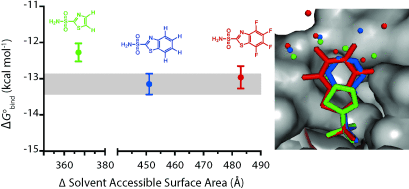
It's the water that matters. Pairs of benzo- and perfluorobenzoarylsulfonamide ligands bind to human carbonic anhydrase with a conserved binding geometry, an enthalpy-driven binding, and indistinguishable binding affinities (see picture). These data support the pervasive theory that the lock-and-key model disregards an important component of binding: the water, which fills the binding pocket of the protein and surrounds the ligand.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201301813_sm_miscellaneous_information.pdf1.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1W. Blokzijl, J. B. F. N. Engberts, Angew. Chem. 1993, 105, 1610–1650; Angew. Chem. Int. Ed. Engl. 1993, 32, 1545–1579.
- 2N. T. Southall, K. A. Dill, A. D. J. Haymet, J. Phys. Chem. B 2002, 106, 521–533.
- 3P. W. Snyder, M. R. Lockett, D. T. Moustakas, G. M. Whitesides, Eur. Phys. J. Spec. Top. 2013, DOI: .
- 4P. W. Snyder, J. Mecinovic, D. T. Moustakas, S. W. Thomas, M. Harder, E. T. Mack, M. R. Lockett, A. Heroux, W. Sherman, G. M. Whitesides, Proc. Natl. Acad. Sci. USA 2011, 108, 17889–17894.
- 5C. Tanford, Proc. Natl. Acad. Sci. USA 1979, 76, 4175–4176.
- 6N. V. Prabhu, K. A. Sharp, Annu. Rev. Phys. Chem. 2005, 56, 521–548.
- 7W. Kauzmann, Annu. Rev. Phys. Chem. 1957, 8, 413–438.
- 8W. P. Jencks, Catalysis in Chemistry and Enzymology, Dover, New York, 1987.
- 9S. W. Homans, Drug Discovery Today 2007, 12, 534–539.
- 10J. Mecinović, P. W. Snyder, K. A. Mirica, S. Bai, E. T. Mack, R. L. Kwant, D. T. Moustakas, A. Héroux, G. M. Whitesides, J. Am. Chem. Soc. 2011, 133, 14017–14026.
- 11For a complete listing of references on enthalpy-dominated hydrophobic effects in protein–ligand binding see the Supporting Information.
- 12J. D. Dunitz, ChemBioChem 2004, 5, 614–621.
- 13M. Salwiczek, E. K. Nyakatura, U. I. M. Gerling, S. Ye, B. Koksch, Chem. Soc. Rev. 2012, 41, 2135–2171.
- 14J. C. Biffinger, H. W. Kim, S. G. DiMagno, ChemBioChem 2004, 5, 622–627.
- 15A. Bondi, J. Phys. Chem. 1964, 68, 441–451.
- 16V. M. Krishnamurthy, G. K. Kaufman, A. R. Urbach, I. Gitlin, K. L. Gudiksen, D. B. Weibel, G. M. Whitesides, Chem. Rev. 2008, 108, 946–1051.
- 17E. A. Meyer, R. K. Castellano, F. Diederich, Angew. Chem. 2003, 115, 1244–1287; Angew. Chem. Int. Ed. 2003, 42, 1210–1250.
- 18For a more complete listing of references on enthalpy-entropy compensation in protein–ligand binding see the Supporting Information.
- 19A. Cornish-Bowden, J. Biosci. 2002, 27, 121–126.
- 20P. D. Lyne, M. L. Lamb, J. C. Saeh, J. Med. Chem. 2006, 49, 4805–4808.
- 21E. Freire, Drug Discovery Today 2008, 13, 869–874.
- 22R. Abel, N. K. Salam, J. Shelley, R. Farid, R. A. Friesner, W. Sherman, ChemMedChem 2011, 6, 1049–1066.
- 23P. R. Connelly, Structure-Based Drug Design: Thermodynamics, Modeling and Strategy, Springer, Berlin, 1997.