Towards First Principles Calculation of Electron Impact Mass Spectra of Molecules†
Corresponding Author
Prof. Dr. Stefan Grimme
Mulliken Center for Theoretical Chemistry, Institut für Physikalische und Theoretische Chemie der Universität Bonn, Beringstrasse 4, 53115 Bonn (Germany)
Mulliken Center for Theoretical Chemistry, Institut für Physikalische und Theoretische Chemie der Universität Bonn, Beringstrasse 4, 53115 Bonn (Germany)Search for more papers by this authorCorresponding Author
Prof. Dr. Stefan Grimme
Mulliken Center for Theoretical Chemistry, Institut für Physikalische und Theoretische Chemie der Universität Bonn, Beringstrasse 4, 53115 Bonn (Germany)
Mulliken Center for Theoretical Chemistry, Institut für Physikalische und Theoretische Chemie der Universität Bonn, Beringstrasse 4, 53115 Bonn (Germany)Search for more papers by this authorThis work was supported by the Fonds der Chemischen Industrie and the DFG in the framework of the SFB 813 (“Chemistry at Spin-Centers”). I thank S. Ehrlich, G. Brandenburg, Dr. A. Hansen, Dr. H. Luftmann, Prof. C. Schalley, Prof. F. Neese, Prof. S. D. Peyerimhoff, Prof. H. Dreeskamp, Prof. M. Allan, Prof. M. Elstner, and Prof. W. Thiel for helpful discussions and J. Mekelburger for technical support.
Graphical Abstract
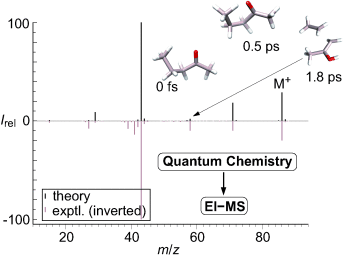
The routine calculation of EI mass spectra is based on a combination of fast quantum chemical methods, molecular dynamics, and the stochastic preparation of “hot” primary ions. All basic elementary processes are considered with minor empiricism and realistic potential free energy surfaces are employed. Reasonable spectra are generated along with detailed information on the corresponding decomposition and reaction mechanisms.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201300158_sm_miscellaneous_information.pdf349.2 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Computational Spectroscopy (Ed.: ), Wiley-VCH, Weinheim, 2010.
- 2J. Gross, Mass Spectrometry—A Textbook, Springer, Weinheim, 2011.
10.1007/978-3-642-10711-5 Google Scholar
- 3F. W. McLafferty, F. Turecek, Interpretation of Mass Spectra, University Science Books, Sausalito, 1993.
- 4L. Drahos, V. Karoly, J. Mass Spectrom. 2001, 36, 237–263.
- 5
- 5aH. Ichikawa, M. Ogata, Bull. Chem. Soc. Jpn. 1973, 46, 1873–1874;
- 5bR. Herzschuh, P. Schröder, M. Scholz, Int. J. Mass Spectrom. Ion Phys. 1981, 38, 79–90;
- 5cI. Mayer, A. Gömöry, J. Mol. Struct. (THEOCHEM) 1994, 311, 331–341;
10.1016/S0166-1280(09)80070-5 Google Scholar
- 5dI. Mayer, A. Gömöry, Chem. Phys. Lett. 2001, 344, 553–564;
- 5eI. P. Csonka, B. Paizs, G. Lendvay, S. Suhai, Rapid Commun. Mass Spectrom. 2000, 14, 417–431.
10.1002/(SICI)1097-0231(20000331)14:6<417::AID-RCM885>3.0.CO;2-J CAS PubMed Web of Science® Google Scholar
- 6
- 6aL. Radom, W. J. Bouma, R. H. Nobes, B. F. Yates, Pure. Appl. Chem. 1984, 56, 1831–1842;
- 6bM. Semialjac, J. Loos, D. Schröder, H. Schwarz, Int. J. Mass Spectrom. 2002, 214, 129–154;
- 6cW. E. Vallejo Narváez, P. V. Bacca Villota, E. A. Solano Espinoza, J. Phys. Chem. A 2012, 116, 12136–12147;
- 6dA. K. Gosh, A. Chattopadhyay, A. Mukhopadhyay, T. Chakraborty, Chem. Phys. Lett. 2013, DOI: .
- 7
- 7aD. Marx, J. Hutter, Ab Initio Molecular Dynamics, Cambridge University Press, Cambridge, 2009;
10.1017/CBO9780511609633 Google Scholar
- 7bB. Kirchner, P. J. di Dio, J. Hutter, Top. Curr. Chem. 2012, 307, 109–154.
- 8
- 8aN. D. Mermin, Phys. Rev. A 1965, 137, 1441–1443;
- 8bR. W. Warren, B. U. Dunlap, Chem. Phys. Lett. 1996, 262, 384–392;
- 8cA. D. Rabuck, G. E. Scuseria, J. Chem. Phys. 1999, 110, 695–700.
- 9
- 9aH. M. Rosenstock, M. B. Wallstein, A. L. Wahrhaftig, H. Eyring, Proc. Natl. Acad. Sci. USA 1952, 38, 667–668;
- 9bW. Frost, Theory of Unimolecular Reactions, Academic Press, New York, 1973;
- 9cM. Vestal, J. H. Futrell, J. Chem. Phys. 1970, 70, 978–988.
- 10
- 10aJ. F. Ying, H. Zhu, C. P. Mathers, B. N. Gover, M. P. Banjavcic, Y. Zheng, C. E. Brion, K. T. Leung, J. Chem. Phys. 1993, 98, 4512–4519;
- 10bW. N. Pang, R. C. Shang, J. F. Gao, N. F. Gao, X. J. Chen, M. S. Deleuze, Chem. Phys. Lett. 1998, 296, 605–610;
- 10cI. V. Litvinyuk, Y. Zheng, C. E. Brion, Chem. Phys. 2000, 261, 289–300.
- 11W. Weber, W. Thiel, Theor. Chem. Acc. 2000, 103, 495–506.
- 12M. Elstner, D. Porezag, G. Jungnickel, J. Elsner, M. Haugk, T. Frauenheim, S. Suhai, G. Seifert, Phys. Rev. B 1998, 58, 7260–7268.
- 13M. Gaus, A. Goez, M. Elstner, J. Chem. Theory Comput. 2013, 9, 338–354.
- 14S. Grimme, J. Antony, S. Ehrlich, H. Krieg, J. Chem. Phys. 2010, 132, 154104.
- 15
- 15aJ. P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett. 1996, 77, 3865–3868, erratum: J. P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett. 1997, 78, 1396;
- 15bC. Adamo, V. Barone, J. Chem. Phys. 1999, 110, 6158–6170.
- 16L. Goerigk, S. Grimme, Phys. Chem. Chem. Phys. 2011, 13, 6670–6688.
- 17S. Grimme, QCEIMS—a general program to compute EI-MS with quantum chemistry, version 2.17, University of Bonn, 2013.
- 18
- 18aF. Neese, ORCA—an ab initio, density functional and semiempirical program package, Verion 2.9 (Rev. 0), Max Planck Institute for Bioinorganic Chemistry, Germany, 2011;
- 18bF. Neese, WIREs Comput. Mol. Sci. 2012, 2, 73–78;
- 18cT. Frauenheim, DFTB+ (Density Functional based Tight Binding) 2008, see http://www.dftb.org/;
- 18dMNDO2005 Version 7.0, W. Thiel, MPI für Kohlenforschung, Mülheim, Germany.
- 19NIST Standard Reference Database, Release September 2012. Editor: Russell D. Johnson III. See http://webbook.nist.gov/chemistry/(accessed November 2012).
- 20G. Remberg, E. Remberg, M. Spiteller-Friedmann, G. Spiteller, Org. Mass Spectrom. 1968, 1, 87–113.
- 21The error (too intense C2H5+ and C3H7+ signals) appears for most tested QC methods and it hence seems unlikely that it is caused by wrong PES. At present we have no further explanation for the observation but cannot exclude errors from the IEE distribution model for this sensitive property.
- 22On a parallel computer with 400 common CPUs, the semiempirical calculation of an EI-MS for a medium-sized molecule takes a few hours of real-time (a few hundred hours of CPU time). DFT calculations at the PBE0/SVx level take about 100 times longer, corresponding to several days of real-time.
- 23See http://www.thch.uni-bonn.de/tc/downloads/movies/.
- 24The relevant maximum reaction time in the spectrometer is on the order of several ns to ms so that very slow reactions can contribute in principle. It is currently not clear how important this contribution is for a larger molecule. While the ms scale cannot be reached by the proposed theory, three very long simulations with a maximum MD time of 1 ns were conducted at the semiempirical level (see the Supporting Information) and some changes compared to the standard cut-off (5–10 ps) were observed. There seems to be, however, some redundancy with the IEE used; in other words, the results of longer simulations with smaller IEE are similar to those from shorter run times with higher IEE. In any case according to these preliminary tests it seems that reactions occurring in the 10–100 ps range are of small to medium importance in some systems but that the ms regime likely is unimportant. Investigations in the 0.1–1 ns range require huge amounts of computer resources but can be conducted for medium-sized molecules with OM2 or DFTB3. Note that absolute rate constants in an EI-MS experiment have apparently never been measured directly. Often cited slow rates (ns to ms range) were deduced from experimental relative ion yields and theory (QET) under the assumption of relatively small IEE (e.g. for propane see Ref. [9c]). Because according to newer (e,2e)-spectroscopy data (see for example, Ref. [10b] for the case of butane) the actual IEE is higher than originally assumed, a ps time-scale as considered here seems to be realistic.