Highly Active Mononuclear NAC–Gold(I) Catalysts†
M. Sc. Maria Camila Blanco Jaimes
Organisch-Chemisches Institut, Ruprecht-Karls-Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany) http://www.hashmi.de
Search for more papers by this authorDipl.-Chem. Constantin R. N. Böhling
Organisch-Chemisches Institut, Ruprecht-Karls-Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany) http://www.hashmi.de
Search for more papers by this authorDr. Juan Manuel Serrano-Becerra
Organisch-Chemisches Institut, Ruprecht-Karls-Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany) http://www.hashmi.de
Search for more papers by this authorCorresponding Author
Prof. Dr. A. Stephen K. Hashmi
Organisch-Chemisches Institut, Ruprecht-Karls-Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany) http://www.hashmi.de
Chemistry Department, Faculty of Science, King Abdulaziz University,Jeddah 21589, Saudi Arabia
Organisch-Chemisches Institut, Ruprecht-Karls-Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany) http://www.hashmi.deSearch for more papers by this authorM. Sc. Maria Camila Blanco Jaimes
Organisch-Chemisches Institut, Ruprecht-Karls-Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany) http://www.hashmi.de
Search for more papers by this authorDipl.-Chem. Constantin R. N. Böhling
Organisch-Chemisches Institut, Ruprecht-Karls-Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany) http://www.hashmi.de
Search for more papers by this authorDr. Juan Manuel Serrano-Becerra
Organisch-Chemisches Institut, Ruprecht-Karls-Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany) http://www.hashmi.de
Search for more papers by this authorCorresponding Author
Prof. Dr. A. Stephen K. Hashmi
Organisch-Chemisches Institut, Ruprecht-Karls-Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany) http://www.hashmi.de
Chemistry Department, Faculty of Science, King Abdulaziz University,Jeddah 21589, Saudi Arabia
Organisch-Chemisches Institut, Ruprecht-Karls-Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany) http://www.hashmi.deSearch for more papers by this authorM.C.B.J. and J.M.S.-B. are grateful to the DAAD for fellowships. Gold salts were generously donated by Umicore AG & Co. KG. NAC=N-acyclic carbene.
Graphical Abstract
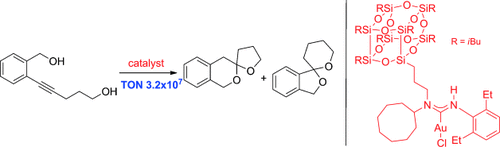
One gold is enough: Mononuclear, homogeneous gold catalysts displayed excellent catalytic activity on the order of that previously reported for gold nanoparticle catalysts and surface-bound gold complexes. In the model synthesis of spiro compounds (see picture) extremely high turnover numbers were achieved with only 0.000001 mol % of a silsesquioxane-substituted catalyst.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201210351_sm_miscellaneous_information.pdf1.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For recent general reviews on gold catalysis:
- 1aN. Krause, C. Winter, Chem. Rev. 2011, 111, 1994–2009;
- 1bA. S. K. Hashmi, M. Rudolph, Chem. Commun. 2011, 47, 6536–6544;
- 1cD. J. Gorin, B. D. Sherry, F. D. Toste, Chem. Rev. 2008, 108, 3351–3378;
- 1dE. Jiménez-Núnez, A. M. Echavarren, Chem. Commun. 2007, 333–346;
- 1eA. Fürstner, P. W. Davies, Angew. Chem. 2007, 119, 3478–3519;
10.1002/ange.200604335 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 3410–3449;
- 1fA. S. K. Hashmi, G. J. Hutchings, Angew. Chem. 2006, 118, 8064–8105;
10.1002/ange.200602454 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 7896–7936.
- 2A. S. K. Hashmi, Angew. Chem. 2010, 122, 5360–5369;
10.1002/ange.200907078 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 5232–5241.
- 3
- 3aA. S. K. Hashmi, M. Rudolph, Chem. Soc. Rev. 2008, 37, 1766–1775;
- 3bA. Fürstner, Chem. Soc. Rev. 2009, 38, 3208–3221;
- 3cA. S. K. Hashmi, M. Rudolph, Chem. Soc. Rev. 2012, 41, 2448–2462.
- 4A. S. K. Hashmi, L. Schwarz, J.-H. Choi, T. M. Frost, Angew. Chem. 2000, 112, 2382–2385;
10.1002/1521-3757(20000703)112:13<2382::AID-ANGE2382>3.0.CO;2-R Google ScholarAngew. Chem. Int. Ed. 2000, 39, 2285–2288.10.1002/1521-3773(20000703)39:13<2285::AID-ANIE2285>3.0.CO;2-F CAS PubMed Web of Science® Google Scholar
- 5J. H. Teles, S. Brode, M. Chabanas, Angew. Chem. 1998, 110, 1475–1478;
10.1002/(SICI)1521-3757(19980518)110:10<1475::AID-ANGE1475>3.0.CO;2-L Google ScholarAngew. Chem. Int. Ed. 1998, 37, 1415–1418.10.1002/(SICI)1521-3773(19980605)37:10<1415::AID-ANIE1415>3.0.CO;2-N CAS PubMed Web of Science® Google Scholar
- 6E. Mizushima, K. Sato, T. Hayashi, M. Tanaka, Angew. Chem. 2002, 114, 4745–4747;
10.1002/1521-3757(20021202)114:23<4745::AID-ANGE4745>3.0.CO;2-S Google ScholarAngew. Chem. Int. Ed. 2002, 41, 4563–4565.10.1002/1521-3773(20021202)41:23<4563::AID-ANIE4563>3.0.CO;2-U CAS PubMed Web of Science® Google Scholar
- 7N. Marion, R. S. Ramón, S. P. Nolan, J. Am. Chem. Soc. 2009, 131, 448–449.
- 8M. Comotti, C. D. Pina, R. Matarrese, M. Rossi, Angew. Chem. 2004, 116, 5936–5939;
10.1002/ange.200460446 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 5812–5815.
- 9M. Bouhrara, E. Jeanneau, L. Veyre, C. Copéret, C. Thieuleux, Dalton Trans. 2011, 40, 2995–2999.
- 10
- 10aJ. Oliver-Meseguer, J. R. Cabrero-Antonino, I. Domínguez, A. Leyva-Pérez, A. Corma, Science 2012, 338, 1452–1455;
- 10bfor a discussion, see: A. S. K. Hashmi, Science 2012, 338, 1434–1434.
- 11C. M. Krauter, A. S. K. Hashmi, M. Pernpointner, ChemCatChem 2010, 2, 1226–1230.
- 12A. S. K. Hashmi, T. M. Frost, J. W. Bats, J. Am. Chem. Soc. 2000, 122, 11553–11554.
- 13A. S. K. Hashmi, T. Hengst, C. Lothschütz, F. Rominger, Adv. Synth. Catal. 2010, 352, 1315–1337.
- 14C.-Y. Zhou, P. W. H. Chan, C.-M. Che, Org. Lett. 2006, 8, 325–328.
- 15
- 15aC. Bartolomé, Z. Ramiro, D. Garca-Cuadrado, P. Pérez-Galán, M. Raducan, C. Bour, A. M. Echavarren, P. Espinet, Organometallics 2010, 29, 951–956;
- 15bA. S. K. Hashmi, C. Lothschütz, C. Böhling, F. Rominger, Organometallics 2011, 30, 2411–2417.
- 16A. S. K. Hashmi, C. Lothschütz, C. Böhling, T. Hengst, C. Hubbert, F. Rominger, Adv. Synth. Catal. 2010, 352, 3001–3012.
- 17
- 17aA. S. K. Hashmi, A. Loos, A. Littmann, I. Braun, J. Knight, S. Doherty, F. Rominger, Adv. Synth. Catal. 2009, 351, 576–582;
- 17bS. Doherty, J. G. Knight, A. S. K. Hashmi, C. H. Smyth, N. A. B. Ward, K. J. Robson, S. Tweedley, R. W. Harrington, W. Clegg, Organometallics 2010, 29, 4139–4147;
- 17cA. S. K. Hashmi, A. Loos, S. Doherty, J. G. Knight, K. J. Robson, F. Rominger, Adv. Synth. Catal. 2011, 353, 749–759;
- 17dS. Doherty, C. H. Smyth, J. G. Knight, A. S. K. Hashmi, Nat. Protoc. 2012, 7, 1870–1883.
- 18For an excellent review, see: H. C. L. Abbenhuis, Chem. Eur. J. 2000, 6, 25–32.
10.1002/(SICI)1521-3765(20000103)6:1<25::AID-CHEM25>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar
- 19N. Mézailles, L. Ricard, F. Gagosz, Org. Lett. 2005, 7, 4133–4136.
- 20
- 20aB. Messerle, K. Q. Vuong, Organometallics 2007, 26, 3031–3040;
- 20bJ. H. H. Ho, S. W. S. Choy, S. A. Macgregor, B. A. Messerle, Organometallics 2011, 30, 5978–5984.
- 21The stock solutions were prepared by stepwise dilution in volumentric flasks. According to the analysis by ICP (inductively coupled plasma), for example, the lowest concentration sample, which was 0.000001 M in catalyst (which is 1.3764 mg L−1 of catalyst and should correspond to 196.97 μg L−1 of gold), showed 188 μg L−1 (the detection limit of the instrument was 1.7 μg L−1, measuring limit 5.3 μg L−1); when another 100 μg L−1 was added to this sample, the value measured as expected was 291 μg L−1.
- 22A. S. K. Hashmi, M. C. Blanco, E. Kurpejovic, W. Frey, J. W. Bats, Adv. Synth. Catal. 2006, 348, 709–713.
- 23In a number of reactions gold and silver are known to be active, see: A. S. K. Hashmi in Silver in Organic Chemistry (Ed.: ), Wiley, Hoboken, 2010, pp. 357–379.
10.1002/9780470597521.ch12 Google Scholar
- 24With 0.01 mol % as well as 0.1 mol % of p-toluenesulfonic acid even after 24 h no conversion could be detected under conditions identical to those of the gold-catalyzed case.