A Self-Assembled Multiporphyrin Cage Complex through Three Different Zinc(II) Center Formation under Well-Balanced Aqueous Conditions†
Takashi Nakamura
Department of Chemistry, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan)
Search for more papers by this authorDr. Hitoshi Ube
Department of Chemistry, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan)
Search for more papers by this authorDr. Motoo Shiro
Rigaku Corporation, 3-9-12 Matsubaracho, Akishima, Tokyo 196-8666 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Mitsuhiko Shionoya
Department of Chemistry, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan)
Department of Chemistry, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan)Search for more papers by this authorTakashi Nakamura
Department of Chemistry, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan)
Search for more papers by this authorDr. Hitoshi Ube
Department of Chemistry, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan)
Search for more papers by this authorDr. Motoo Shiro
Rigaku Corporation, 3-9-12 Matsubaracho, Akishima, Tokyo 196-8666 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Mitsuhiko Shionoya
Department of Chemistry, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan)
Department of Chemistry, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan)Search for more papers by this authorThis research was supported by Global COE Program and KAKENHI from the Japan Society for the Promotion of Science (JSPS) and MEXT (Japan). T. Nakamura thanks JSPS Research Fellowship for Young Scientists.
Graphical Abstract
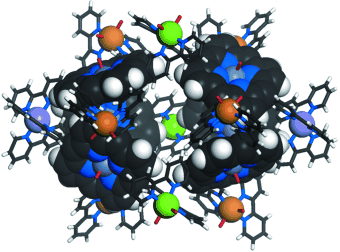
Construction of a self-assembled cage complex through three different ZnII centers is achieved using a Zn porphyrin ligand with four 2,2′-bipyridin-5-yl (bpy) groups. The multiporphyrin cage encapsulates guest molecules unsymmetrically by π–π interactions. Well-balanced aqueous conditions, which allow the formation of both tris(bpy) and hydrated bis(bpy) ZnII units, result in the unsymmetrical yet well-defined supramolecular structure.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201208040_sm_miscellaneous_information.pdf9.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. W. Steed, J. L. Atwood, Supramolecular Chemistry, 2nd ed., Wiley, New York, 2009;
10.1002/9780470740880 Google Scholar
- 1bP. J. Stang, J. Am. Chem. Soc. 2012, 134, 11829–11830.
- 2R. Chakrabarty, P. S. Mukherjee, P. J. Stang, Chem. Rev. 2011, 111, 6810–6918.
- 3
- 3aJ. Rebek, Jr., Angew. Chem. 2005, 117, 2104–2115;
10.1002/ange.200462839 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 2068–2078;
- 3bG. V. Oshovsky, D. N. Reinhoudt, M. Verboom, Angew. Chem. 2007, 119, 2418–2445;
10.1002/ange.200602815 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 2366–2393.
- 4
- 4aD. M. Vriezema, M. C. Aragons, J. A. A. W. Elemans, J. J. L. M. Cornelissen, A. E. Rowan, R. J. M. Nolte, Chem. Rev. 2005, 105, 1445–1490;
- 4bM. Yoshizawa, J. K. Klosterman, M. Fujita, Angew. Chem. 2009, 121, 3470–3490; Angew. Chem. Int. Ed. 2009, 48, 3418–3438;
- 4cM. J. Wiester, P. A. Ulmann, C. A. Mirkin, Angew. Chem. 2011, 123, 118–142; Angew. Chem. Int. Ed. 2011, 50, 114–137.
- 5
- 5aA. Scarso, H. Onagi, J. Rebek, Jr., J. Am. Chem. Soc. 2004, 126, 12728–12729;
- 5bS. Harthong, B. Dubessy, J. Vachon, C. Aronica, J.-C. Mulatier, J.-P. Dutasta, J. Am. Chem. Soc. 2010, 132, 15637–15643.
- 6
- 6aM. Kawano, Y. Kobayashi, T. Ozeki, M. Fujita, J. Am. Chem. Soc. 2006, 128, 6558–6559;
- 6bP. Mal, N. Breiner, K. Rissanen, J. R. Nitschke, Science 2009, 324, 1697–1699.
- 7
- 7aX. Sun, D. W. Johnson, D. L. Caulder, K. N. Raymond, E. H. Wong, J. Am. Chem. Soc. 2001, 123, 2752–2763;
- 7bS. Hiraoka, Y. Sakata, M. Shionoya, J. Am. Chem. Soc. 2008, 130, 10058–10059;
- 7cK. Mahata, M. L. Saha, M. Schmittel, J. Am. Chem. Soc. 2010, 132, 15933–15935;
- 7dX.-P. Zhou, J. Liu, S.-Z. Zhan, J.-R. Yang, D. Li, K.-M. Ng, R. W.-Y. Sun, C.-M. Che, J. Am. Chem. Soc. 2012, 134, 8042–8045;
- 7eM. M. J. Smulders, A. Jiménez, J. R. Nitschke, Angew. Chem. 2012, 124, 6785–6789;
10.1002/ange.201202050 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 6681–6685.
- 8
- 8aI. S. Tidmarsh, T. B. Faust, H. Adams, L. P. Harding, L. Russo, W. Clegg, M. D. Ward, J. Am. Chem. Soc. 2008, 130, 15167–15175;
- 8bA. Stephenson, S. P. Argent, T. Riis-Johannessen, I. S. Tidmarsh, M. D. Ward, J. Am. Chem. Soc. 2011, 133, 858–870;
- 8cF. Li, J. K. Clegg, L. F. Lindoy, R. B. Macquart, G. V. Meehan, Nat. Commun. 2011, 2, 205.
- 9
- 9aJ. E. Kuder, J. M. Pochan, S. R. Turner, D. F. Hinman, J. Electrochem. Soc. 1978, 125, 1750–1758;
- 9bS. C. Zimmerman, K. W. Saionz, Z. Zeng, Proc. Natl. Acad. Sci. USA 1993, 90, 1190–1193.
- 10K. M. Kadish, K. M. Smith, R. Guilard, Handbook of Porphyrin Science, Vol. 1–25, World Scientific, Singapore, 2010–2012.
- 11
- 11aR. Takahashi, Y. Kobuke, J. Am. Chem. Soc. 2003, 125, 2372–2373;
- 11bN. Fujita, K. Biradha, M. Fujita, S. Sakamoto, K. Yamaguchi, Angew. Chem. 2001, 113, 1768–1771;
Angew. Chem. Int. Ed. 2001, 40, 1718–1721;
10.1002/1521-3773(20010504)40:9<1718::AID-ANIE17180>3.0.CO;2-7 CAS PubMed Web of Science® Google Scholar
- 11cM. L. Merlau, M. P. Mejia, S. T. Nguyen, J. T. Hupp, Angew. Chem. 2001, 113, 4369–4372;
10.1002/1521-3757(20011119)113:22<4369::AID-ANGE4369>3.0.CO;2-C Google ScholarAngew. Chem. Int. Ed. 2001, 40, 4239–4242;10.1002/1521-3773(20011119)40:22<4239::AID-ANIE4239>3.0.CO;2-E CAS PubMed Web of Science® Google Scholar
- 11dJ. Aimi, Y. Nagamine, A. Tsuda, A. Muranaka, M. Uchiyama, T. Aida, Angew. Chem. 2008, 120, 5231–5234;
10.1002/ange.200801332 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 5153–5156;
- 11eM. C. O’Sullivan, J. K. Sprafke, D. V. Kondratuk, C. Rinfray, T. D. W. Claridge, A. Saywell, M. O. Blunt, J. N. O’Shea, P. H. Beton, M. Malfois, H. L. Anderson, Nature 2011, 469, 72–75;
- 11fI. Beletskaya, V. S. Tyurin, A. Y. Tsivadze, R. Guilard, C. Stern, Chem. Rev. 2009, 109, 1659–1713.
- 12W. Meng, B. Breiner, K. Rissanen, J. D. Thoburn, J. K. Clegg, J. R. Nitschke, Angew. Chem. 2011, 123, 3541–3545; Angew. Chem. Int. Ed. 2011, 50, 3479–3483.
- 13G. H. Eom, H. M. Park, M. Y. Hyun, S. P. Jang, C. Kim, J. H. Lee, S. J. Lee, S.-J. Kim, Y. Kim, Polyhedron 2011, 30, 1555–1564.
- 14Crystal data for 2: C394H304Cl12F66N72O122S22Zn17, FW=11495.26, red blocks, 0.29×0.22×0.16 mm3, trigonal, space group R32 (no. 155), a=24.812(1), c=79.373(3) Å, V=42320(3) Å3, Z=3, T=93 K, λ(MoKα)=0.71075 Å, 2θmax=37.68°, Friedel pairs 3302, R1=0.0875, wR2=0.2418 (after SQUEEZE), GOF=1.062, Flack Parameter 0.50(3), largest diff. peak and hole 0.74/−0.40 e Å−3. See the Supporting Information for details. CCDC 881253 (1) and 881254 (2) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.