Asymmetric Hydrogenation of α,α′-Disubstituted Cycloketones through Dynamic Kinetic Resolution: An Efficient Construction of Chiral Diols with Three Contiguous Stereocenters†
Chong Liu
State Key Laboratory and Institute of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (China) http://zhou.nankai.edu.cn
Search for more papers by this authorCorresponding Author
Prof. Jian-Hua Xie
State Key Laboratory and Institute of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (China) http://zhou.nankai.edu.cn
State Key Laboratory and Institute of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (China) http://zhou.nankai.edu.cnSearch for more papers by this authorYa-Li Li
State Key Laboratory and Institute of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (China) http://zhou.nankai.edu.cn
Search for more papers by this authorJi-Qiang Chen
State Key Laboratory and Institute of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (China) http://zhou.nankai.edu.cn
Search for more papers by this authorCorresponding Author
Prof. Qi-Lin Zhou
State Key Laboratory and Institute of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (China) http://zhou.nankai.edu.cn
State Key Laboratory and Institute of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (China) http://zhou.nankai.edu.cnSearch for more papers by this authorChong Liu
State Key Laboratory and Institute of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (China) http://zhou.nankai.edu.cn
Search for more papers by this authorCorresponding Author
Prof. Jian-Hua Xie
State Key Laboratory and Institute of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (China) http://zhou.nankai.edu.cn
State Key Laboratory and Institute of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (China) http://zhou.nankai.edu.cnSearch for more papers by this authorYa-Li Li
State Key Laboratory and Institute of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (China) http://zhou.nankai.edu.cn
Search for more papers by this authorJi-Qiang Chen
State Key Laboratory and Institute of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (China) http://zhou.nankai.edu.cn
Search for more papers by this authorCorresponding Author
Prof. Qi-Lin Zhou
State Key Laboratory and Institute of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (China) http://zhou.nankai.edu.cn
State Key Laboratory and Institute of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (China) http://zhou.nankai.edu.cnSearch for more papers by this authorWe thank the National Natural Science Foundation of China, the National Basic Research Program of China (2012CB821600), and the “111” project (B06005) of the Ministry of Education of China for financial support.
Graphical Abstract
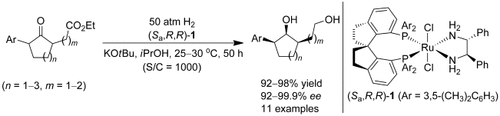
Chiral diols with three contiguous stereocenters were synthesized by a highly enantioselective ruthenium-catalyzed asymmetric hydrogenation of racemic α,α′-disubstituted cycloketones involving dynamic kinetic resolution. This new catalytic asymmetric method provides a concise route to the alkaloid (+)-γ-lycorane.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201207561_sm_miscellaneous_information.pdf9.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews, see:
- 1aT. Ohkuma, M. Kitamura, R. Noyori in Catalytic Asymmetric Synthesis, 2nd ed. ), Wiley-Interscience, New York, 2000, p. 1;
10.1002/0471721506.ch1 Google Scholar
- 1bH.-U. Blaser, B. Pugin, F. Spindler, Applied Homogeneous Catalysis with Organometallic Compounds, 2nd ed., Wiley-VCH, Weinheim, 2000, chap. 3.3.1;
- 1cW. Tang, X. Zhang, Chem. Rev. 2003, 103, 3029;
- 1dJ.-H. Xie, S.-F. Zhu, Q.-L. Zhou, Chem. Rev. 2011, 111, 3340;
- 1eD. J. Ager, A. H. M. de Vries, J. G. de Vries, Chem. Soc. Rev. 2012, 41, 3340;
- 1fJ.-H. Xie, Q.-L. Zhou, Acta Chim. Sin. 2012, 70, 1427.
- 2For reviews, see:
- 2aJ. P. Genet, Acc. Chem. Res. 2003, 36, 908;
- 2bH. Pellissier, Tetrahedron 2008, 64, 1563;
- 2cH. Pellissier, Tetrahedron 2011, 67, 3769.
- 3
- 3aR. Noyori, M. Tokunaga, M. Kitamura, Bull. Chem. Soc. Jpn. 1995, 68, 36;
- 3bM. Scalone, P. Waldmeier, Org. Process Res. Dev. 2003, 7, 418;
- 3cT. Ohkuma, J. Li, R. Noyori, Synlett 2004, 1383;
- 3dJ.-H. Xie, S. Liu, X.-H. Huo, X. Cheng, H.-F. Duan, B.-M. Fan, L.-X. Wang, Q.-L. Zhou, J. Org. Chem. 2005, 70, 2967;
- 3eS. Liu, J.-H. Xie, L.-X. Wang, Q.-L. Zhou, Angew. Chem. 2007, 119, 7650; Angew. Chem. Int. Ed. 2007, 46, 7506;
- 3fN. Arai, H. Ooka, K. Azuma, T. Yabuuchi, N. Kurono, T. Inoue, T. Ohkuma, Org. Lett. 2007, 9, 939;
- 3gC.-Y. Chen, L. F. Frey, S. Shultz, D. J. Wallace, K. Marcantonio, J. F. Payack, E. Vazquez, S. A. Springfield, G. Zhou, P. Liu, G. R. Kieczykowski, A. M. Chen, B. D. Phenix, U. Singh, J. Strine, B. Izzo, S. W. Krska, Org. Process Res. Dev. 2007, 11, 616;
- 3hJ.-H. Xie, S. Liu, W.-L. Kong, W.-J. Bai, X.-C. Wang, L.-X. Wang, Q.-L. Zhou, J. Am. Chem. Soc. 2009, 131, 4222;
- 3iS. Liu, J.-H. Xie, W. Li, W.-L. Kong, L.-X. Wang, Q.-L. Zhou, Org. Lett. 2009, 11, 4994;
- 3jJ.-H. Xie, W.-L. Kong, X.-C. Wang, W.-J. Bai, L.-X. Wang, Q.-L. Zhou, Front. Chem. China 2009, 4, 299;
10.1007/s11458-009-0093-9 Google Scholar
- 3kT. Hibino, K. Makino, T. Sugiyama, Y. Hamada, ChemCatChem 2009, 1, 237;
- 3lW.-J. Bai, J.-H. Xie, Y.-L. Li, S. Liu, Q.-L. Zhou, Adv. Synth. Catal. 2010, 352, 81.
- 4Noyori et al. have reported a nonasymmetric hydrogenation of 2,6-dimethylcyclohexanone with RuCl2PPh3/(CH2NH2)2 catalyst that produces the corresponding cis,cis-cyclohexanol with 98.7 % cis,cis-selectivity. T. Ohkuma, H. Ooka, M. Yamakawa, T. Ikariya, R. Noyori, J. Org. Chem. 1996, 61, 4872.
- 5(+)-γ-Lycorane was obtained by Kotera in his degradation studies of lycorine, see: K. Kotera, Tetrahedron 1961, 12, 248.
- 6
- 6aV. Prelog, K. Wiesner, H. G. Khorana, G. W. Kenner, Helv. Chim. Acta 1949, 59, 453;
- 6bH. Iida, T. Takarai, C. Kibayashi, J. Org. Chem. 1978, 43, 975;
- 6cY.-M. Zhao, P. Gu, Y.-Q. Tu, H.-J. Zhang, Q.-W. Zhang, C.-A. Fan, J. Org. Chem. 2010, 75, 5289.
- 7
- 7aJ.-H. Xie, Z.-T. Zhou, W.-L. Kong, Q.-L. Zhou, J. Am. Chem. Soc. 2007, 129, 1868;
- 7bJ.-H. Xie, Q.-L. Zhou, Acc. Chem. Res. 2008, 41, 581;
- 7cZ.-T. Zhou, J.-H. Xie, Q.-L. Zhou, Adv. Synth. Catal. 2009, 351, 363;
- 7dJ.-Q. Chen, J.-H. Xie, D.-H. Bao, S. Liu, Q.-L. Zhou, Org. Lett. 2012, 14, 2714;
- 7eL.-J. Cheng, J.-H. Xie, L.-X. Wang, Q.-L. Zhou, Adv. Synth. Catal. 2012, 354, 1105.
- 8For recent examples of catalytic hydrogenation of esters, see:
- 8aJ. Zhang, G. Leitus, Y. Ben-David, D. Milstein, Angew. Chem. 2006, 118, 1131; Angew. Chem. Int. Ed. 2006, 45, 1113;
- 8bL. A. Saudan, C. M. Saudan, C. Debieux, P. Wyss, Angew. Chem. 2007, 119, 7617;
10.1002/ange.200701015 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 7473;
- 8cW. Kuriyama, Y. Ino, O. Ogata, N. Sayo, T. Saito, Adv. Synth. Catal. 2010, 352, 92;
- 8dE. Fogler, E. Balaraman, Y. Ben-David, G. Leitus, J. W. Shimon, D. Milstein, Organometallics 2011, 30, 3826;
- 8eM. Ito, T. Ootsuka, R. Watari, A. Shiibashi, T. Ikariya, J. Am. Chem. Soc. 2011, 133, 4240;
- 8fD. Spasyuk, S. Smith, D. G. Gusev, Angew. Chem. 2012, 124, 2826;
10.1002/ange.201108956 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 2772.
- 9The asymmetric hydrogenation of racemic 2 a with catalysts (Sa,R,R)-1 a and (Sa,R,R)-1 b also gave chiral diol cis,cis-3 a in high yields (96 %) and cis,cis-selectivity with 95 % ee and 96 % ee, respectively.
- 10For recent examples of the total synthesis of optically active (+)-γ-lycorane, see:
- 10aH. Yoshizaki, H. Satoh, Y. Sato, S. Nukui, M. Shibasaki, M. Mori, J. Org. Chem. 1995, 60, 2016;
- 10bM. Ikeda, S. Ohtani, T. Sato, H. Ishibashi, Synthesis 1998, 1803;
- 10cM. G. Banwell, J. E. Harvey, D. C. Hockless, J. Org. Chem. 2000, 65, 4241;
- 10dL. Dong, Y.-J. Xu, L.-F. Cun, X. Cui, A.-Q. Mi, Y.-Z. Jiang, L.-Z. Gong, Org. Lett. 2005, 7, 4285;
- 10eB. D. Chapsal, I. Ojima, Org. Lett. 2006, 8, 1395;
- 10fK. Tomooka, M. Suzuki, K. Uehara, M. Shimada, T. Akiyama, Synlett 2008, 2518; for the total synthesis of optically active (−)-γ-lycorane, see:
- 10gH. Fujioka, K. Murai, Y. Ohba, H. Hirose, Y. Kita, Chem. Commun. 2006, 832;
- 10hS. A. A. El Bialy, Nat. Prod. Res. 2008, 22, 1176.