Light-Induced Modular Ligation of Conventional RAFT Polymers†
Kim K. Oehlenschlaeger
Preparative Macromolecular Chemistry, Institut für Technische Chemie und Polymerchemie, Karlsruhe Institute of Technology (KIT), Engesserstrasse 18, 76128 Karlsruhe (Deutschland) http://www.macroarc.de
These authors contributed equally to this work.
Search for more papers by this authorJan O. Mueller
Preparative Macromolecular Chemistry, Institut für Technische Chemie und Polymerchemie, Karlsruhe Institute of Technology (KIT), Engesserstrasse 18, 76128 Karlsruhe (Deutschland) http://www.macroarc.de
These authors contributed equally to this work.
Search for more papers by this authorNiklas B. Heine
Preparative Macromolecular Chemistry, Institut für Technische Chemie und Polymerchemie, Karlsruhe Institute of Technology (KIT), Engesserstrasse 18, 76128 Karlsruhe (Deutschland) http://www.macroarc.de
Search for more papers by this authorMathias Glassner
Preparative Macromolecular Chemistry, Institut für Technische Chemie und Polymerchemie, Karlsruhe Institute of Technology (KIT), Engesserstrasse 18, 76128 Karlsruhe (Deutschland) http://www.macroarc.de
Search for more papers by this authorDr. Nathalie K. Guimard
Preparative Macromolecular Chemistry, Institut für Technische Chemie und Polymerchemie, Karlsruhe Institute of Technology (KIT), Engesserstrasse 18, 76128 Karlsruhe (Deutschland) http://www.macroarc.de
Search for more papers by this authorDr. Guillaume Delaittre
Preparative Macromolecular Chemistry, Institut für Technische Chemie und Polymerchemie, Karlsruhe Institute of Technology (KIT), Engesserstrasse 18, 76128 Karlsruhe (Deutschland) http://www.macroarc.de
Search for more papers by this authorDr. Friedrich G. Schmidt
Evonik Industries AG, Paul-Baumann-Strasse 1, 45764 Marl (Deutschland)
Search for more papers by this authorCorresponding Author
Prof. Dr. Christopher Barner-Kowollik
Preparative Macromolecular Chemistry, Institut für Technische Chemie und Polymerchemie, Karlsruhe Institute of Technology (KIT), Engesserstrasse 18, 76128 Karlsruhe (Deutschland) http://www.macroarc.de
Preparative Macromolecular Chemistry, Institut für Technische Chemie und Polymerchemie, Karlsruhe Institute of Technology (KIT), Engesserstrasse 18, 76128 Karlsruhe (Deutschland) http://www.macroarc.deSearch for more papers by this authorKim K. Oehlenschlaeger
Preparative Macromolecular Chemistry, Institut für Technische Chemie und Polymerchemie, Karlsruhe Institute of Technology (KIT), Engesserstrasse 18, 76128 Karlsruhe (Deutschland) http://www.macroarc.de
These authors contributed equally to this work.
Search for more papers by this authorJan O. Mueller
Preparative Macromolecular Chemistry, Institut für Technische Chemie und Polymerchemie, Karlsruhe Institute of Technology (KIT), Engesserstrasse 18, 76128 Karlsruhe (Deutschland) http://www.macroarc.de
These authors contributed equally to this work.
Search for more papers by this authorNiklas B. Heine
Preparative Macromolecular Chemistry, Institut für Technische Chemie und Polymerchemie, Karlsruhe Institute of Technology (KIT), Engesserstrasse 18, 76128 Karlsruhe (Deutschland) http://www.macroarc.de
Search for more papers by this authorMathias Glassner
Preparative Macromolecular Chemistry, Institut für Technische Chemie und Polymerchemie, Karlsruhe Institute of Technology (KIT), Engesserstrasse 18, 76128 Karlsruhe (Deutschland) http://www.macroarc.de
Search for more papers by this authorDr. Nathalie K. Guimard
Preparative Macromolecular Chemistry, Institut für Technische Chemie und Polymerchemie, Karlsruhe Institute of Technology (KIT), Engesserstrasse 18, 76128 Karlsruhe (Deutschland) http://www.macroarc.de
Search for more papers by this authorDr. Guillaume Delaittre
Preparative Macromolecular Chemistry, Institut für Technische Chemie und Polymerchemie, Karlsruhe Institute of Technology (KIT), Engesserstrasse 18, 76128 Karlsruhe (Deutschland) http://www.macroarc.de
Search for more papers by this authorDr. Friedrich G. Schmidt
Evonik Industries AG, Paul-Baumann-Strasse 1, 45764 Marl (Deutschland)
Search for more papers by this authorCorresponding Author
Prof. Dr. Christopher Barner-Kowollik
Preparative Macromolecular Chemistry, Institut für Technische Chemie und Polymerchemie, Karlsruhe Institute of Technology (KIT), Engesserstrasse 18, 76128 Karlsruhe (Deutschland) http://www.macroarc.de
Preparative Macromolecular Chemistry, Institut für Technische Chemie und Polymerchemie, Karlsruhe Institute of Technology (KIT), Engesserstrasse 18, 76128 Karlsruhe (Deutschland) http://www.macroarc.deSearch for more papers by this authorC.B.-K. is grateful for continued support from Evonik Industries and the Karlsruhe Institute of Technology (KIT) in the context of the Excellence Initiative for leading German universities as well as the Ministry of Science and Arts of the state of Baden-Württemberg.
Graphical Abstract
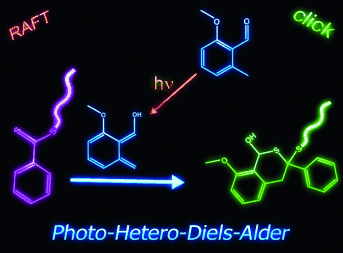
Making light work of RAFT conjugation: A non-activated RAFT agent at the end of RAFT polymers can readily be coupled with ortho-quinodimethanes (photoenols) in a photo-triggered Diels–Alder reaction under mild conditions without catalyst (see scheme). The method is universal and opens the door for the conjugation of a large number of RAFT-prepared polymers with photoenol-functionalized (macro)molecules. (RAFT=reversible addition-fragmentation chain transfer.)
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201206905_sm_miscellaneous_information.pdf759.7 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aC. Barner-Kowollik, F. E. Du Prez, P. Espeel, C. J. Hawker, T. Junkers, H. Schlaad, W. Van Camp, Angew. Chem. 2011, 123, 61–64; Angew. Chem. Int. Ed. 2011, 50, 60–62;
- 1bH. C. Kolb, M. G. Finn, K. B. Sharpless, Angew. Chem. 2001, 113, 2056–2075;
Angew. Chem. Int. Ed. 2001, 40, 2004–2021;
10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5 CAS PubMed Web of Science® Google Scholar
- 1cC. J. Hawker, K. L. Wooley, Science 2005, 309, 1200–1205;
- 1dC. Barner-Kowollik, A. J. Inglis, Macromol. Chem. Phys. 2009, 210, 987–992.
- 2W. H. Binder, R. Sachsenhofer, Macromol. Rapid Commun. 2008, 29, 952–981.
- 3
- 3aM. A. Tasdelen, Polym. Chem. 2011, 2, 2133–2145;
- 3bA. J. Inglis, M. H. Stenzel, C. Barner-Kowollik, Macromol. Rapid Commun. 2009, 30, 1792–1798.
- 4
- 4aK. L. Heredia, Z. P. Tolstyka, H. D. Maynard, Macromolecules 2007, 40, 4772–4779;
- 4bT. Pauloehrl, G. Delaittre, M. Bruns, M. Meißler, H. G. Börner, M. Bastmeyer, C. Barner-Kowollik, Angew. Chem. 2012, 124, 9316–9319;
10.1002/ange.201202684 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 9181–9184.
- 5M. Dietrich, G. Delaittre, J. P. Blinco, A. J. Inglis, M. Bruns, C. Barner-Kowollik, Adv. Funct. Mater. 2012, 22, 304–312.
- 6
- 6aL. Nebhani, S. Sinnwell, A. J. Inglis, M. H. Stenzel, C. Barner-Kowollik, L. Barner, Macromol. Rapid Commun. 2008, 29, 1431–1437;
- 6bS. Sinnwell, A. J. Inglis, T. P. Davis, M. H. Stenzel, C. Barner-Kowollik, Chem. Commun. 2008, 2052–2054;
- 6cS. Sinnwell, A. J. Inglis, M. H. Stenzel, C. Barner-Kowollik, Macromol. Rapid Commun. 2008, 29, 1090–1096;
- 6dS. Sinnwell, M. Lammens, M. H. Stenzel, F. E. Du Prez, C. Barner-Kowollik, J. Polym. Sci. Part A 2009, 47, 2207–2213;
- 6eM. Glassner, G. Delaittre, M. Kaupp, J. P. Blinco, C. Barner-Kowollik, J. Am. Chem. Soc. 2012, 134, 7274–7277.
- 7
- 7aK. Matyjaszewski, Macromolecules 2012, 45, 4015–4039;
- 7bJ. Nicolas, Y. Guillaneuf, C. Lefay, D. Bertin, D. Gigmes, B. Charleux, Prog. Polym. Sci. 2012, DOI: .
- 8
- 8aC. Boyer, M. H. Stenzel, T. P. Davis, J. Polym. Sci. Part A 2011, 49, 551–595;
- 8bC. Barner-Kowollik, Handbook of RAFT Polymerization, Wiley-VCH, Weinheim, 2008.
10.1002/9783527622757 Google Scholar
- 9
- 9aA. J. Inglis, S. Sinnwell, M. H. Stenzel, C. Barner-Kowollik, Angew. Chem. 2009, 121, 2447–2450;
10.1002/ange.200805993 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 2411–2414;
- 9bL. Nebhani, S. Sinnwell, C. Y. Lin, M. L. Coote, M. H. Stenzel, C. Barner-Kowollik, J. Polym. Sci. Part A 2009, 47, 6053–6071.
- 10
- 10aM. Glassner, K. K. Oehlenschlaeger, T. Gruendling, C. Barner-Kowollik, Macromolecules 2011, 44, 4681–4689;
- 10bT. Gruendling, K. K. Oehlenschlaeger, E. Frick, M. Glassner, C. Schmid, C. Barner-Kowollik, Macromol. Rapid Commun. 2011, 32, 807–812.
- 11T. Pauloehrl, G. Delaittre, V. Winkler, A. Welle, M. Bruns, H. G. Börner, A. M. Greiner, M. Bastmeyer, C. Barner-Kowollik, Angew. Chem. 2012, 124, 1096–1099;
10.1002/ange.201107095 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 1071–1074.
- 12
- 12aP. G. Sammes, Tetrahedron 1976, 32, 405–422;
- 12bJ. L. Charlton, M. M. Alauddin, Tetrahedron 1987, 43, 2873–2889;
- 12cJ. L. Segura, N. Martín, Chem. Rev. 1999, 99, 3199–3246.
- 13
- 13aG. Moad, E. Rizzardo, S. H. Thang, Aust. J. Chem. 2005, 58, 379–410;
- 13bG. Moad, E. Rizzardo, S. H. Thang, Aust. J. Chem. 2006, 59, 669–692;
- 13cG. Moad, E. Rizzardo, S. H. Thang, Aust. J. Chem. 2009, 62, 1402–1472.
- 14S. Perrier, C. Barner-Kowollik, J. F. Quinn, P. Vana, T. P. Davis, Macromolecules 2002, 35, 8300–8306.
- 15
- 15aL. Charles, M. Lejars, A. Margaillan, C. Bressy, Int. J. Mass Spectrom. 2012, 311, 31–39;
- 15bA. T. Jackson, S. E. Slade, J. H. Scrivens, Int. J. Mass Spectrom. 2004, 238, 265–277.
- 16C. Barner-Kowollik, Macromol. Rapid Commun. 2009, 30, 1625–1631.
- 17G. Moad, J. Chiefari, Y. K. Chong, J. Krstina, R. T. A. Mayadunne, A. Postma, E. Rizzardo, S. H. Thang, Polym. Int. 2000, 49, 993–1001.
- 18The solutions were deoxygenated by three consecutive freeze–pump–thaw cycles. It is noteworthy that the deoxygenation method has an influence on the overall kinetics. Indeed, the execution of three freeze–pump–thaw cycles yields faster reactions than those after deoxygenation by nitrogen bubbling.