Iridium-Catalyzed Asymmetric Hydrogenation of Pyridinium Salts†
Zhi-Shi Ye
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences (CAS), 457 Zhongshan Road, Dalian 116023 (China) http://www.lac.dicp.ac.cn/
Search for more papers by this authorMu-Wang Chen
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences (CAS), 457 Zhongshan Road, Dalian 116023 (China) http://www.lac.dicp.ac.cn/
Search for more papers by this authorQing-An Chen
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences (CAS), 457 Zhongshan Road, Dalian 116023 (China) http://www.lac.dicp.ac.cn/
Search for more papers by this authorLei Shi
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences (CAS), 457 Zhongshan Road, Dalian 116023 (China) http://www.lac.dicp.ac.cn/
Search for more papers by this authorYing Duan
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences (CAS), 457 Zhongshan Road, Dalian 116023 (China) http://www.lac.dicp.ac.cn/
Search for more papers by this authorCorresponding Author
Prof. Yong-Gui Zhou
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences (CAS), 457 Zhongshan Road, Dalian 116023 (China) http://www.lac.dicp.ac.cn/
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences (CAS), 457 Zhongshan Road, Dalian 116023 (China) http://www.lac.dicp.ac.cn/Search for more papers by this authorZhi-Shi Ye
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences (CAS), 457 Zhongshan Road, Dalian 116023 (China) http://www.lac.dicp.ac.cn/
Search for more papers by this authorMu-Wang Chen
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences (CAS), 457 Zhongshan Road, Dalian 116023 (China) http://www.lac.dicp.ac.cn/
Search for more papers by this authorQing-An Chen
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences (CAS), 457 Zhongshan Road, Dalian 116023 (China) http://www.lac.dicp.ac.cn/
Search for more papers by this authorLei Shi
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences (CAS), 457 Zhongshan Road, Dalian 116023 (China) http://www.lac.dicp.ac.cn/
Search for more papers by this authorYing Duan
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences (CAS), 457 Zhongshan Road, Dalian 116023 (China) http://www.lac.dicp.ac.cn/
Search for more papers by this authorCorresponding Author
Prof. Yong-Gui Zhou
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences (CAS), 457 Zhongshan Road, Dalian 116023 (China) http://www.lac.dicp.ac.cn/
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences (CAS), 457 Zhongshan Road, Dalian 116023 (China) http://www.lac.dicp.ac.cn/Search for more papers by this authorFinancial support was provided by the National Natural Science Foundation of China (21032003, 20921092 and 21125208) and the National Basic Research Program of China (2010CB833300).
Graphical Abstract
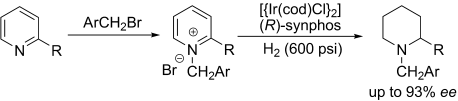
Highly efficient iridium-catalyzed asymmetric hydrogenations of simple 2-substituted pyridinium salts gives the chiral 2-substituted piperidines with up to 93 % ee (see picture; cod=1,5-cyclooctadiene; synphos=(5,6),(5′,6′)-bis(ethylenedioxy)-2,2′-bis(diphenylphosphino)-1,1′-biphenyl). The key feature of this strategy is the activation of simple pyridines as the pyridinium salts, thus eliminating substrate inhibition and enhancing the reactivity.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201205187_sm_miscellaneous_information.pdf3.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews on the hydrogenation of aromatic compounds, see:
- 1aY.-G. Zhou, Acc. Chem. Res. 2007, 40, 1357;
- 1bD.-S. Wang, Q.-A. Chen, S.-M. Lu, Y.-G. Zhou, Chem. Rev. 2012, 112, 2557;
- 1cF. Glorius, Org. Biomol. Chem. 2005, 3, 4171;
- 1dR. Kuwano, Heterocycles 2008, 76, 909;
- 1eN. Fleury-Brégeot, V. de La Fuente, S. Castillón, C. Claver, ChemCatChem 2010, 2, 1346.
- 2For selected works on asymmetric reduction of quinolines, see:
- 2aW.-B. Wang, S.-M. Lu, P.-Y. Yang, X.-W. Han, Y.-G. Zhou, J. Am. Chem. Soc. 2003, 125, 10536;
- 2bM. Rueping, A. P. Antonchick, T. Theissmann, Angew. Chem. 2006, 118, 3765;
10.1002/ange.200600191 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 3683;
- 2cQ.-S. Guo, D.-M. Du, J. Xu, Angew. Chem. 2008, 120, 771; Angew. Chem. Int. Ed. 2008, 47, 759;
- 2dH. Zhou, Z. Li, Z. Wang, T. Wang, L. Xu, Y. He, Q.-H. Fan, J. Pan, L. Gu, A. S. C. Chan, Angew. Chem. 2008, 120, 8592; Angew. Chem. Int. Ed. 2008, 47, 8464;
- 2eC. Wang, C. Li, X. Wu, A. Pettman, J. Xiao, Angew. Chem. 2009, 121, 6646; Angew. Chem. Int. Ed. 2009, 48, 6524;
- 2fT. Wang, L.-G. Zhuo, Z. Li, F. Chen, Z. Ding, Y. He, Q.-H. Fan, J. Xiang, Z.-X. Yu, A. S. C. Chan, J. Am. Chem. Soc. 2011, 133, 9878;
- 2gQ.-A. Chen, K. Gao, Y. Duan, Z.-S. Ye, L. Shi, Y. Yang, Y.-G. Zhou, J. Am. Chem. Soc. 2012, 134, 2442.
- 3For recent works on asymmetric reduction of isoquinolines, see:
- 3aS.-M. Lu, Y.-Q. Wang, X.-W. Han, Y.-G. Zhou, Angew. Chem. 2006, 118, 2318; Angew. Chem. Int. Ed. 2006, 45, 2260;
- 3bL. Shi, Z.-S. Ye, L.-L. Cao, R.-N. Guo, Y. Hu, Y.-G. Zhou, Angew. Chem. 2012, 124, 8411; Angew. Chem. Int. Ed. 2012, 51, 8286.
- 4For selected works on asymmetric reduction of quinoxalines, see:
- 4aC. Bianchini, P. Barbaro, G. Scapacci, E. Farnetti, M. Graziani, Organometallics 1998, 17, 3308;
- 4bW. Tang, L. Xu, Q.-H. Fan, J. Wang, B. Fan, Z. Zhou, K.-H. Lam, A. S. C. Chan, Angew. Chem. 2009, 121, 9299; Angew. Chem. Int. Ed. 2009, 48, 9135;
- 4cQ.-A. Chen, D.-S. Wang, Y.-G. Zhou, Y. Duan, H.-J. Fan, Y. Yang, Z. Zhang, J. Am. Chem. Soc. 2011, 133, 6126;
- 4dM. Rueping, F. Tato, F. R. Schoepke, Chem. Eur. J. 2010, 16, 2688.
- 5For selected works on asymmetric reduction of indoles, see:
- 5aR. Kuwano, K. Sato, T. Kurokawa, D. Karube, Y. Ito, J. Am. Chem. Soc. 2000, 122, 7614;
- 5bD.-S. Wang, Q.-A. Chen, W. Li, C.-B. Yu, Y.-G. Zhou, X. Zhang, J. Am. Chem. Soc. 2010, 132, 8909.
- 6For work on asymmetric reduction of pyrroles, see:
- 6aR. Kuwano, M. Kashiwabara, M. Ohsumi, H. Kusano, J. Am. Chem. Soc. 2008, 130, 808;
- 6bD.-S. Wang, Z.-S. Ye, Q.-A. Chen, Y.-G. Zhou, C.-B. Yu, H.-J. Fan, Y. Duan, J. Am. Chem. Soc. 2011, 133, 8866.
- 7For work on asymmetric reduction of furans, see:
- 7aS. Kaiser, S. P. Smidt, A. Pfaltz, Angew. Chem. 2006, 118, 5318;
10.1002/ange.200601529 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 5194;
- 7bN. Ortega, S. Urban, B. Beiring, F. Glorius, Angew. Chem. 2012, 124, 1742;
10.1002/ange.201107811 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 1710.
- 8For work on asymmetric reduction of imidazoles and axazoles, see: R. Kuwano, N. Kameyama, R. Ikeda, J. Am. Chem. Soc. 2011, 133, 7312.
- 9For work on asymmetric reduction of the carbocyclic ring of aromatic compounds, see:
- 9aS. Urban, N. Ortega, F. Glorius, Angew. Chem. 2011, 123, 3887; Angew. Chem. Int. Ed. 2011, 50, 3803;
- 9bR. Kuwano, R. Morioka, M. Kashiwabara, N. Kameyama, Angew. Chem. 2012, 124, 4212;
10.1002/ange.201201153 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 4136.
- 10
- 10aM. Studer, C. Wedemeyer-Exl, F. Spindler, H.-U. Blaser, Monatsh. Chem. 2000, 131, 1335;
- 10bS. A. Raynor, J. M. Thomas, R. Raja, B. F. G. Johnson, R. G. Bell, M. D. Mantle, Chem. Commun. 2000, 1925;
- 10cR. Raja, J. M. Thomas, J. Mol. Catal. A 2002, 181, 3.
- 11A. Lei, M. Chen, M. He, X. Zhang, Eur. J. Org. Chem. 2006, 4343.
- 12M. Rueping, A. P. Antonchick, Angew. Chem. 2007, 119, 4646;
10.1002/ange.200701158 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 4562.
- 13
- 13aX.-B. Wang, W. Zeng, Y.-G. Zhou, Tetrahedron Lett. 2008, 49, 4922;
- 13bW.-J. Tang, J. Tan, L.-J. Xu, K.-H. Lam, Q.-H. Fan, A. S. C. Chan, Adv. Synth. Catal. 2010, 352, 1055;
- 13cW.-J. Tang, Y. W. Sun, L. J. Xu, T.-L. Wang, Q.-H. Fan, K.-H. Lam, A. S. C. Chan, Org. Biomol. Chem. 2010, 8, 3464; for the diastereoselective heterogeneous hydrogenation of auxiliary-substituted pyridines, see:
- 13dF. Glorius, N. Spielkamp, S. Holle, R. Goddard, C. W. Lehmann, Angew. Chem. 2004, 116, 2910; Angew. Chem. Int. Ed. 2004, 43, 2850.
- 14
- 14aC. Y. Legault, A. B. Charette, J. Am. Chem. Soc. 2005, 127, 8966;
- 14bC. Y. Legault, A. B. Charette, P. G. Cozzi, Heterocycles 2008, 76, 1271.
- 15
- 15aM. Rubiralta, E. Giralt, A. Diez, Piperidine: Structure, Preparation and Synthetic Applications of Piperidine and its Derivatives, Elsevier, Amsterdam 1991; for recent review, see:
- 15bM. Ahamed, M. H. Todd, Eur. J. Org. Chem. 2010, 5935;
- 15cP. D. Bailey, P. A. Millwood, P. D. Smith, Chem. Commun. 1998, 633;
- 15dM. G. P. Buffat, Tetrahedron 2004, 60, 1701;
- 15eC. Escolano, M. Amat, J. Bosch, Chem. Eur. J. 2006, 12, 8198;
- 15fF.-X. Felpin, J. Lebreton, Eur. J. Org. Chem. 2003, 3693;
- 15gJ. P. A. Harrity, O. Provoost, Org. Biomol. Chem. 2005, 3, 1349;
- 15hH. P. Husson, J. Royer, Chem. Soc. Rev. 1999, 28, 383;
- 15iA. Mitchenson, A. Nadin, J. Chem. Soc. Perkin Trans. 1 2000, 2862;
- 15jD. M. Stout, A. I. Meyers, Chem. Rev. 1982, 82, 223;
- 15kP. Weintraub, Tetrahedron 2003, 59, 2953;
- 15lS. Laschat, T. Dickner, Synthesis 2000, 1781.
- 16
- 16aJ. A. Bull, J. J. Mousseau, G. Pelletier, A. B. Charette, Chem. Rev. 2012, 112, 2642;
- 16bJ. Deng, J. Zhu, J. Wu, J. Liao, Synlett 2006, 2059;
- 16cJ. Wu, F. Wang, Y. Ma, X. Cui, L. Cun, J. Zhu, J. Deng, B. Yu, Chem. Commun. 2006, 1766;
- 16dJ. Szawkało, S. J. Czarnocki, A. Zawadzka, K. Wojtasiewicz, A. Leniewski, J. K. Maurin, Z. Czarnocki, J. Drabowicz, Tetrahedron: Asymmetry 2007, 18, 406;
- 16eJ. Szawkało, A. Zawadzka, K. Wojtasiewicz, A. Leniewski, J. Drabowicz, Z. Czarnocki, Tetrahedron: Asymmetry 2005, 16, 3619;
- 16fG. Hou, F. Gosselin, W. Li, C. McWilliams, Y. Sun, M. Weisel, P. D. O’Shea, C.-Y. Chen, I. W. Davies, X. Zhang, J. Am. Chem. Soc. 2009, 131, 9882;
- 16gG. Hou, W. Li, M. Ma, X. W. Zhang, X. Zhang, J. Am. Chem. Soc. 2010, 132, 12844;
- 16hG. Hou, R. Tao, Y. Sun, X. Zhang, F. Gosselin, J. Am. Chem. Soc. 2010, 132, 2124;
- 16iD.-S. Wang, Y.-G. Zhou, Tetrahedron Lett. 2010, 51, 3014;
- 16jH. Tadaoka, D. Cartigny, T. Nagano, T. Gosavi, T. Ayad, J. P. Genêt, T. Ohshima, V. Ratovelomanana-Vidal, K. Mashima, Chem. Eur. J. 2009, 15, 9990;
- 16kD.-S. Wang, J. Tang, Y.-G. Zhou, M.-W. Chen, C.-B. Yu, Y. Duan, G.-F. Jiang, Chem. Sci. 2011, 2, 803;
- 16lY. Duan, M.-W. Chen, Z.-S. Ye, D.-S. Wang, Q.-A. Chen, Y.-G. Zhou, Chem. Eur. J. 2011, 17, 7193;
- 16mY. Duan, M.-W. Chen, Q.-A. Chen, C.-B. Yu, Y.-G. Zhou, Org. Biomol. Chem. 2012, 10, 1235;
- 16nY.-Q. Cheng, Z. Bian, C.-Q. Kang, H.-Q. Guo, L.-X. Gao, Tetrahedron: Asymmetry 2008, 19, 1572;
- 16oA. Noole, K. Lippur, A. Metsala, M. Lopp, T. Kanger, J. Org. Chem. 2010, 75, 1313.
- 17
- 17aD. Xiao, B. J. Lavey, A. Palani, C. Wang, R. G. Aslanian, J. A. Kozlowski, N.-Y. Shih, A. T. McPhail, G. P. Randolph, J. E. Lachowicz, R. A. Duffy, Tetrahedron Lett. 2005, 46, 7653;
- 17bN. S. Sheikh, D. Leonori, G. Barker, J. D. Firth, K. R. Campos, A. J. H. M. Meijer, P. O’Brien, I. Coldham, J. Am. Chem. Soc. 2012, 134, 5300.
- 18
- 18aT. K. Beng, R. E. Gawley, Org. Lett. 2011, 13, 394;
- 18bF. Chen, Z. Ding, J. Qin, T. Wang, Y. He, Q.-H. Fan, Org. Lett. 2011, 13, 4348;
- 18cJ. C. A. Hunt, P. Laurent, C. J. Moody, J. Chem. Soc. Perkin Trans. 1 2002, 2378.