CH⋅⋅⋅O Hydrogen Bonding Induced Triazole Foldamers: Efficient Halogen Bonding Receptors for Organohalogens†
Dr. Li-Yan You
State Key Laboratory of Bioorganic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)
These authors contributed equally to this project.
Search for more papers by this authorDr. Shi-Gui Chen
State Key Laboratory of Bioorganic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)
These authors contributed equally to this project.
Search for more papers by this authorCorresponding Author
Prof. Xin Zhao
State Key Laboratory of Bioorganic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)
State Key Laboratory of Bioorganic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)Search for more papers by this authorProf. Yi Liu
The Molecular Foundry, Lawrence Berkeley National Laboratory, One Cyclotron Road, Berkeley, CA 94720 (USA)
Search for more papers by this authorDr. Wen-Xian Lan
State Key Laboratory of Bioorganic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)
Search for more papers by this authorYing Zhang
Chemistry of Department, Fudan University, 220 Handan Road, Shanghai 200433 (China)
Search for more papers by this authorProf. Hao-Jie Lu
Chemistry of Department, Fudan University, 220 Handan Road, Shanghai 200433 (China)
Search for more papers by this authorProf. Chun-Yang Cao
State Key Laboratory of Bioorganic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)
Search for more papers by this authorCorresponding Author
Prof. Zhan-Ting Li
State Key Laboratory of Bioorganic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)
Chemistry of Department, Fudan University, 220 Handan Road, Shanghai 200433 (China)
State Key Laboratory of Bioorganic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)Search for more papers by this authorDr. Li-Yan You
State Key Laboratory of Bioorganic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)
These authors contributed equally to this project.
Search for more papers by this authorDr. Shi-Gui Chen
State Key Laboratory of Bioorganic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)
These authors contributed equally to this project.
Search for more papers by this authorCorresponding Author
Prof. Xin Zhao
State Key Laboratory of Bioorganic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)
State Key Laboratory of Bioorganic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)Search for more papers by this authorProf. Yi Liu
The Molecular Foundry, Lawrence Berkeley National Laboratory, One Cyclotron Road, Berkeley, CA 94720 (USA)
Search for more papers by this authorDr. Wen-Xian Lan
State Key Laboratory of Bioorganic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)
Search for more papers by this authorYing Zhang
Chemistry of Department, Fudan University, 220 Handan Road, Shanghai 200433 (China)
Search for more papers by this authorProf. Hao-Jie Lu
Chemistry of Department, Fudan University, 220 Handan Road, Shanghai 200433 (China)
Search for more papers by this authorProf. Chun-Yang Cao
State Key Laboratory of Bioorganic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)
Search for more papers by this authorCorresponding Author
Prof. Zhan-Ting Li
State Key Laboratory of Bioorganic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)
Chemistry of Department, Fudan University, 220 Handan Road, Shanghai 200433 (China)
State Key Laboratory of Bioorganic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)Search for more papers by this authorWe thank NSFC (20921091 and 20974118) and STCSM (10J1412200 and 09XD1405300) for financial support.
Graphical Abstract
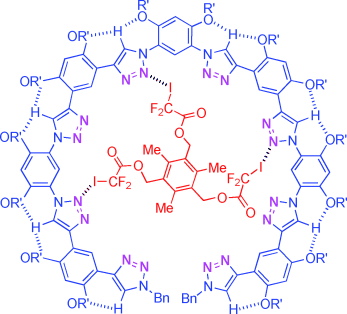
Into the fold: Intramolecular CH⋅⋅⋅O hydrogen bonding has been utilized to create new aromatic triazole foldamers. Remarkably, all the triazole units of the foldamers are guided to orientate inward to form a nitrogen ring. As a result, they can efficiently bind neutral tri- and didentate organohalogens through multiple N⋅⋅⋅X (X=Cl, Br, I) halogen bonds to form stable 1:1 complexes.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201106996_sm_miscellaneous_information.pdf1.7 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Halogen Bonding: Fundamentals and Applications (Eds.: ), Springer, Berlin, 2008.
- 2
- 2aP. Metrangolo, H. Neukirch, T. Pilati, G. Resnati, Acc. Chem. Res. 2005, 38, 386–395;
- 2bP. Metrangolo, F. Meyer, T. Pilati, G. Resnati, G. Terraneo, Angew. Chem. 2008, 120, 6206–6220;
10.1002/ange.200800128 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 6114–6127.
- 3
- 3aM. D. Ward, Dalton Trans. 2010, 39, 8851–8867;
- 3bK. Rissanen, CrystEngComm 2008, 10, 1107–1113;
- 3cM. Fourmigue, Struct. Bonding (Berlin) 2008, 126, 181–207;
- 3dD. W. Bruce, Struct. Bonding (Berlin) 2008, 126, 161–180;
- 3eP. Metrangolo, G. Resnati, T. Pilati, S. Biella, Struct. Bonding (Berlin) 2008, 126, 105–136;
- 3fK. Rissanen, CrystEngComm 2008, 10, 1107–1113;
- 3gM. Fourmigué, Curr. Opin. Solid State Mater. Sci. 2009, 13, 36–45;
- 3hG. Cavallo, P. Metrangolo, T. Pilati, G. Resnati, M. Sansotera, G. Terraneo, Chem. Soc. Rev. 2010, 39, 3772–3783;
- 3iR. Bertani, P. Sgarbossa, A. Venzo, F. Lelj, M. Amati, G. Resnati, T. Pilati, P. Metrangolo, G. Terraneo, Coord. Chem. Rev. 2010, 254, 677–695;
- 3jK. Merz, V. Vasylyeva, CrystEngComm 2010, 12, 3989–4002.
- 4
- 4aA. R. Voth, P. S. Ho, Curr. Top. Med. Chem. 2007, 7, 1336–1348;
- 4bE. Parisini, P. Metrangolo, T. Pilati, G. Resnati, G. Terraneo, Chem. Soc. Rev. 2011, 40, 2267–2278;
- 4cP. Zhou, F. Tian, J. Zou, Z. Shang, Mini-Rev. Med. Chem. 2010, 10, 309–314.
- 5A. Bruckmann, M. A. Pena, C. Bolm, Synlett 2008, 900–902.
- 6
- 6aP. Politzer, P. Lane, M. C. Concha, Y. Ma, J. S. Murray, J. Mol. Model. 2007, 13, 305–311;
- 6bA. Karpfen, Struct. Bonding (Berlin) 2008, 126, 1–15;
- 6cA. C. Legon, Phys. Chem. Chem. Phys. 2010, 12, 7736–7747;
- 6dP. Politzer, J. S. Murray, T. Clark, Phys. Chem. Chem. Phys. 2010, 12, 7748–7757.
- 7
- 7aK. Xu, D. M. Ho, R. A. Pascal, J. Org. Chem. 1995, 60, 7186–7191;
- 7bS. C. Blackstock, J. K. Kochi, J. P. Lorand, J. Org. Chem. 1987, 52, 1451–1460;
- 7cS. Libri, N. A. Jasim, R. N. Perutz, L. Brammer, J. Am. Chem. Soc. 2008, 130, 7842–7844;
- 7dR. Cabot, C. Hunter, Chem. Commun. 2009, 2005–2007;
- 7eM. G. Sarwar, B. Dragisic, L. J. Salsberg, C. Gouliaras, M. S. Taylor, J. Am. Chem. Soc. 2010, 132, 1646–1653.
- 8P. L. Wash, S. Ma, U. Obst, J. Rebek, Jr., J. Am. Chem. Soc. 1999, 121, 7973–7974.
- 9C. A. Hunter, H. L. Anderson, Angew. Chem. 2009, 121, 7624–7636; Angew. Chem. Int. Ed. 2009, 48, 7488–7499.
- 10A. Mulder, J. Huskens, D. N. Reinhoudt, Org. Biomol. Chem. 2004, 2, 3409–3424.
- 11Y.-Y. Zhu, G.-T. Wang, R.-X. Wang, Z.-T. Li, Cryst. Growth Des. 2009, 9, 4778–4783.
- 12D. Zornik, R. M. Meudtner, T. E. Malah, C. M. Thiele, S. Hecht, Chem. Eur. J. 2011, 17, 1473–1484.
- 13Y. Hua, A. H. Flood, Chem. Soc. Rev. 2010, 39, 1262–1271.
- 14aY. Li, A. H. Flood, Angew. Chem. 2008, 120, 2689–2692; Angew. Chem. Int. Ed. 2008, 47, 2649–2652;
- 14bY. Li, M. Pink, J. A. Karty, A. H. Flood, J. Am. Chem. Soc. 2008, 130, 17293–17295;
- 14cY. Li, A. H. Flood, J. Am. Chem. Soc. 2008, 130, 12111–12122;
- 14dS. Lee, Y. Hua, H. Park, A. H. Flood, Org. Lett. 2010, 12, 2100–2102.
- 15aH. Juwarker, J. M. Lenhardt, D. M. Pham, S. L. Craig, Angew. Chem. 2008, 120, 3800–3803;
10.1002/ange.200800548 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 3740–3743;
- 15bH. Juwarker, J. M. Lenhardt, J. C. Castillo, E. Zhao, S. Krishnamurthy, R. M. Jamiolkowski, K.-H. Kim, S. L. Craig, J. Org. Chem. 2009, 74, 8924–8934.
- 16aR. M. Meudtner, S. Hecht, Angew. Chem. 2008, 120, 5004–5008;
10.1002/ange.200800796 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 4926–4930;
- 16bM. Ostermeier, M.-A. Berlin, R. Meudtner, S. Demeshko, F. Meyer, C. Limberg, S. Hecht, Chem. Eur. J. 2010, 16, 10202–10213;
- 16cR. M. Meudtner, S. Hecht, Macromol. Rapid Commun. 2008, 29, 347–351;
- 16dL. Piot, R. M. Meudtner, T. El Malah, S. Hecht, P. Samori, Chem. Eur. J. 2009, 15, 4788–4792.
- 17
- 17aY. Wang, F. Li, Y. Han, F. Wang, H. Jiang, Chem. Eur. J. 2009, 15, 9424–9433;
- 17bY. Wang, J. Xiang, H. Jiang, Chem. Eur. J. 2011, 17, 613–619.
- 18Hydrogen bonded aromatic amide foldamers have been widely utilized for molecular recognition by intermolecular hydrogen bonds, see:
- 18aC. Li, S.-F. Ren, J.-L. Hou, H.-P. Yi, S.-Z. Zhu, X.-K. Jiang, Z.-T. Li, Angew. Chem. 2005, 117, 5871–5875; Angew. Chem. Int. Ed. 2005, 44, 5725–5729;
- 18bJ. Garric, J.-M. Léger, I. Huc, Angew. Chem. 2005, 117, 1990–1994;
10.1002/ange.200462898 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 1954–1958;
- 18cC. Bao, B. Kauffmann, Q. Gan, K. Srinivas, H. Jiang, I. Huc, Angew. Chem. 2008, 120, 4221–4224; Angew. Chem. Int. Ed. 2008, 47, 4153–4156;
- 18dK. Yamato, L. Yuan, W. Feng, A. J. Helsel, A. R. Sanford, J. Zhu, J. Deng, X. C. Zeng, B. Gong, Org. Biomol. Chem. 2009, 7, 3643–3647;
- 18eQ. Gan, Y. Ferrand, C. Bao, B. Kauffmann, A. Grélard, H. Jiang, I. Huc, Science 2011, 331, 1172–1175.
- 19CCDC 846798 (2 a) and 846799 (2 b) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 20
- 20aE. Dimitrijević, O. Kvak, M. S. Taylor, Chem. Commun. 2010, 46, 9025–9027;
- 20bM. G. Sarwar, B. Dragisic, S. Sagoo, M. S. Taylor, Angew. Chem. 2010, 122, 1718–1721;
10.1002/ange.200906488 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 1674–1677;
- 20cM. G. Chudzinski, C. A. McClary, M. S. Taylor, J. Am. Chem. Soc. 2011, 133, 10559–10567.
- 21H. A. Benesi, J. H. Hildebrand, J. Am. Chem. Soc. 1949, 71, 2703–2707.