Catalytic Degenerate and Nondegenerate Oxygen Atom Transfers Employing N2O and CO2 and a MII/MIV Cycle Mediated by Group 6 MIV Terminal Oxo Complexes†
Brendan L. Yonke
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD 20742 (USA)
Search for more papers by this authorJonathan P. Reeds
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD 20742 (USA)
Search for more papers by this authorPeter Y. Zavalij
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD 20742 (USA)
Search for more papers by this authorCorresponding Author
Prof. Lawrence R. Sita
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD 20742 (USA)
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD 20742 (USA)Search for more papers by this authorBrendan L. Yonke
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD 20742 (USA)
Search for more papers by this authorJonathan P. Reeds
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD 20742 (USA)
Search for more papers by this authorPeter Y. Zavalij
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD 20742 (USA)
Search for more papers by this authorCorresponding Author
Prof. Lawrence R. Sita
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD 20742 (USA)
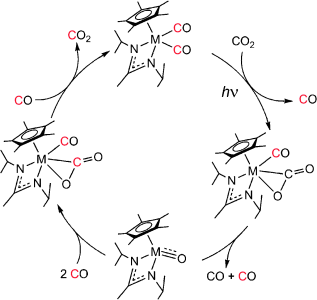
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD 20742 (USA)Search for more papers by this authorFunding for this work was provided by the Department of Energy, Basic Energy Sciences (DE-SC0002217). M=Mo, W.
Graphical Abstract
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201106074_sm_miscellaneous_information.pdf1.9 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1G. Henrici-Olivé, S. Olivé, Angew. Chem. 1974, 86, 1–12; Angew. Chem. Int. Ed. Engl. 1974, 13, 29–38.
- 2aW. B. Tolman, Angew. Chem. 2010, 122, 1034–1041;
10.1002/ange.200905364 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 1018–1024;
- 2bK. H. Theopold, O. M. Reinaud, S. Blanchard, S. Leelasubcharoen, A. Hess, S. Thyagarajan, ACS Symp. Ser. 2002, 823, 75–85;
- 2cX. Yin, J. R. Moss, Coord. Chem. Rev. 1999, 181, 27–59.
- 3 Ullmann’s Encyclopedia of Industrial Chemistry, 7th ed. ), Wiley-VCH, Weinheim, 2011.
- 4
- 4aR. H. Holm, E. I. Solomon, A. Majumdar, A. Tenderholt, Coord. Chem. Rev. 2011, 255, 993–1015;
- 4bC. Lorber, J. P. Donahue, C. A. Goddard, E. Nordlander, R. H. Holm, J. Am. Chem. Soc. 1998, 120, 8102–8112;
- 4cJ. P. Donahue, C. R. Goldsmith, U. Nadiminti, R. H. Holm, J. Am. Chem. Soc. 1998, 120, 12869–12881.
- 5For late transition metal and noble metal catalyzed oxidation of RNC to RNCO employing O2 or CO2 as the oxidants, see:
- 5aS. Otsuka, A. Nakamura, Y. Tatsuno, J. Chem. Soc. Chem. Commun. 1967, 836;
- 5bS. Németh, L. I. Simandi, Inorg. Chim. Acta 1982, 64, L21–L22;
- 5cD. L. DeLaet, R. Del Rosario, P. E. Fanwick, C. P. Kubiak, J. Am. Chem. Soc. 1987, 109, 754–758;
- 5dJ. Wu, P. E. Fanwick, C. P. Kubiak, Organometallics 1987, 6, 1805–1807;
- 5eT. J. Deming, B. M. Novak, J. Am. Chem. Soc. 1993, 115, 9101–9011;
- 5fR. J. Angelici, J. Organomet. Chem. 2008, 693, 847–856, and references therein.
- 6For chemical turnover of a WII/WIV OAT cycle, see: U. Jayarathne, P. Chandrasekaran, H. Jacobsen, J. T. Mague, J. P. Donahue, Dalton Trans. 2010, 39, 9662–9671, and ref. [10].
- 7P. P. Fontaine, B. L. Yonke, P. Y. Zavalij, L. R. Sita, J. Am. Chem. Soc. 2010, 132, 12273–12285.
- 8
- 8aM. R. Smith III, P. T. Matsunaga, R. A. Andersen, J. Am. Chem. Soc. 1993, 115, 7049–7050;
- 8bW. A. Howard, M. Waters, G. Parkin, J. Am. Chem. Soc. 1993, 115, 4917–4918.
- 9Experimental details are provided in the Supporting Information.
- 10
- 10aF. M. Su, C. Cooper, S. J. Geib, A. L. Rheingold, J. M. Mayer, J. Am. Chem. Soc. 1986, 108, 3545–3547;
- 10bJ. C. Bryan, S. J. Geib, A. L. Rheingold, J. M. Mayer, J. Am. Chem. Soc. 1987, 109, 2826–2828;
- 10cK. A. Hall, J. M. Mayer, J. Am. Chem. Soc. 1992, 114, 10402–10411.
- 11We wish to acknowledge the contribution of Wesley Farrell who prepared and isolated an analytically pure and crystalline sample of 7 suitable for single-crystal X-ray analysis.
- 12B. Cordero, V. Gomez, A. E. Platero-Prats, M. Reves, J. Escheverria, E. Cremades, F. Barragan, S. Alvarez, Dalton Trans. 2008, 2832–2838.
- 13K. Iijima, S. Shibata, Chem. Lett. 1972, 1033–1036.
- 14
- 14aN. D. Silavwe, M. Y. Chiang, D. R. Tyler, Inorg. Chem. 1985, 24, 4219–4221;
- 14bN. D. Silavwe, M. R. M. Bruce, C. E. Philbin, D. R. Tyler, Inorg. Chem. 1988, 27, 4669–4676;
- 14cG. T. Baxley, A. A. Avey, T. M. Aukett, D. R. Tyler, Inorg. Chim. Acta 2000, 300, 102–112.
- 15L. Luo, G. Lanza, I. L. Fragala, C. L. Stern, T. J Marks, J. Am. Chem. Soc. 1998, 120, 3111–3122.
- 16
- 16aA. J. Bridgeman, L. Davis, S. J. Dixon, J. C. Green, I. N. Wright, J. Chem. Soc. Dalton Trans. 1995, 1023–1027;
- 16bJ. R. Galsworthy, J. C. Green, M. L. H. Green, M. Müller, J. Chem. Soc. Dalton Trans. 1998, 15–20.
- 17
- 17aW. A. Nugent, B. L. Haymore, Coord. Chem. Rev. 1980, 31, 123–175;
- 17bW. A. Nugent, J. M. Mayer, Metal-Ligand Multiple Bonds, Wiley-Interscience, New York, 1988.
- 18
- 18aM. L. H. Green, A. H. Lynch, M. G. Swanwick, J. Chem. Soc. Dalton Trans. 1972, 1445–1447;
- 18bP. Jernakoff, G. L. Geoffroy, A. L. Rheingold, S. J. Geib, J. Chem. Soc. Chem. Commun. 1987, 1610–1611;
- 18cR. S. Pilato, G. L. Geoffroy, A. L. Rheingold, J. Chem. Soc. Chem. Commun. 1989, 1287–1288;
- 18dR. S. Pilato, D. Rubin, G. L. Geoffroy, A. L. Rheingold, Inorg. Chem. 1990, 29, 1986–1990;
- 18eR. S. Pilato, C. E. Housmekerides, P. Jernakoff, D. Rubin, G. L. Geoffroy, A. L. Rheingold, Organometallics 1990, 9, 2333–2341.
- 19
- 19aG. Parkin, J. E. Bercaw, Polyhedron 1988, 7, 2053–2082;
- 19bG. Parkin, R. E. Marsh, W. P. Schaefer, J. E. Bercaw, Inorg. Chem. 1988, 27, 3262–3264;
- 19cG. Parkin, J. E. Bercaw, J. Am. Chem. Soc. 1989, 111, 391–393;
- 19dG. Parkin, Prog. Inorg. Chem. 1998, 47, 1–165;
- 19eJ. H. Shin, D. G. Churchill, B. M. Bridgewater, K. Pang, G. Parkin, Inorg. Chim. Acta 2006, 359, 2942–2955.
- 20P. Braunstein, D. Nobel, Chem. Rev. 1989, 89, 1927–1945.
- 21F. Minzoni, C. Pelizzi, G. Predieri, J. Organomet. Chem. 1982, 231, C 6-C8.
- 22
- 22aC. M. Giamdomenico, L. H. Hanau, S. J. Lippard, Organometallics. 1982, 1, 142–148;
- 22bW. D. Jones, W. P. Kosar, Organometallics 1986, 5, 1823–1829;
- 22cW. A. Herrmann, B. Menjón, E. Herdtweck, Organometallics 1991, 10, 2134–2141.
- 23Y. Kim, J. Gallucci, A. Wojcicki, J. Am. Chem. Soc. 1990, 112, 8600–8602.
- 24
- 24aP.-F. Fu, M. A. Khan, K. M. Nicholas, J. Am. Chem. Soc. 1992, 114, 6579–6580;
- 24bP.-F. Fu, M. A. Khan, K. M. Nicholas, J. Organomet. Chem. 1996, 506, 49–59;
- 24cT.-F. Wang, C.-C. Hwu, C.-W. Tsai, K.-J. Lin, Organometallics 1997, 16, 3089–3090.
- 25G. Fachinetti, C. Floriani, A. Chiesi-Villa, C. Guastini, J. Am. Chem. Soc. 1979, 101, 1767–1775.
- 26We thank a reviewer for contributing this insight.