Asymmetric Conjugate Addition of Glycine Derivatives under Copper Catalysis†
Dr. Mark Strohmeier
API Chemistry and Analysis, GlaxoSmithKline Pharmaceuticals, King of Prussia, PA (USA)
Search for more papers by this authorKevin Leach
API Chemistry and Analysis, GlaxoSmithKline Pharmaceuticals, King of Prussia, PA (USA)
Search for more papers by this authorCorresponding Author
Dr. Matthew A. Zajac
API Chemistry and Analysis, GlaxoSmithKline Pharmaceuticals, King of Prussia, PA (USA)
API Chemistry and Analysis, GlaxoSmithKline Pharmaceuticals, King of Prussia, PA (USA)Search for more papers by this authorDr. Mark Strohmeier
API Chemistry and Analysis, GlaxoSmithKline Pharmaceuticals, King of Prussia, PA (USA)
Search for more papers by this authorKevin Leach
API Chemistry and Analysis, GlaxoSmithKline Pharmaceuticals, King of Prussia, PA (USA)
Search for more papers by this authorCorresponding Author
Dr. Matthew A. Zajac
API Chemistry and Analysis, GlaxoSmithKline Pharmaceuticals, King of Prussia, PA (USA)
API Chemistry and Analysis, GlaxoSmithKline Pharmaceuticals, King of Prussia, PA (USA)Search for more papers by this authorWe thank Alan Freyer for help with NMR studies, J. J. Conde and Huan Wang for helpful discussions, and Doug Fuerst and Chris Morgan for help with HPLC data.
Graphical Abstract
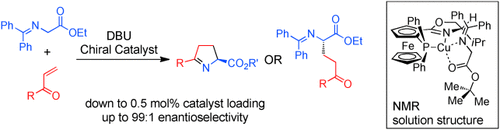
Coppertunity knocks: An inexpensive, practical, and environmentally benign procedure for the enantioselective addition of glycine derivatives to α,β-unsaturated ketones has been developed (see scheme). By modifying the workup, the conjugate addition product or a cyclic imine can be accessed. The solution structure of the catalyst–substrate complex is shown to be key to the overall reaction selectivity.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201105258_sm_miscellaneous_information.pdf18.7 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. van der Werf, R. M. Kellogg, Tetrahedron Lett. 1991, 32, 3727;
- 1bJ. Sisko, J. R. Henry, S. M. Weinreb, J. Org. Chem. 1993, 58, 4945;
- 1cL. E. Overman, J. E. Tellew, J. Org. Chem. 1996, 61, 8338;
- 1dW. H. Pearson in Studies in Natural Product Chemistry, Vol. 1 (Ed.: ), Elsevier, New York, 1998, p. 323;
- 1eD. J. Denhart, D. A. Griffith, C. H. Heathcock, J. Org. Chem. 1998, 63, 9616;
- 1fT. Shibuguchi, H. Mihara, A. Kuramochi, S. Sakuraba, T. Ohshima, M. Shibasaki, Angew. Chem. 2006, 118, 4751;
10.1002/ange.200601722 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 4635;
- 1gT. Arai, A. Mishiro, N. Yokoyama, K. Suzuki, H. Sato, J. Am. Chem. Soc. 2010, 132, 5338.
- 2
- 2aK. V. Gothelf, K. A. Jørgensen, Chem. Rev. 1998, 98, 863;
- 2bI. Coldham, R. Hufton, Chem. Rev. 2005, 105, 2765;
- 2cG. Pandey, P. Banerjee, S. R. Gadre, Chem. Rev. 2006, 106, 4484;
- 2dL. Stanley, M. P. Sibi, Chem. Rev. 2008, 108, 2887.
- 3
- 3aJ. S. Petersen, G. Fels, H. Rapoport, J. Am. Chem. Soc. 1984, 106, 4539;
- 3bZ. Pei, X. Li, K. Longenecker, T. W. von Geldern, P. E. Wiedeman, T. H. Lubben, B. A. Zinker, K. Stewart, S. J. Ballaron, M. A. Stashko, A. K. Mika, D. W. A. Beno, M. Long, H. Wells, A. J. Kempf-Grote, D. J. Madar, T. S. McDermott, L. Bhagavatula, M. G. Fickes, D. Pireh, L. R. Solomon, M. R. Lake, R. Edalji, E. H. Fry, H. L. Sham, J. M. Trevillyan, J. Med Chem. 2006, 49, 3520;
- 3cG. Alvaro, D. Amantini, M. Bergauer, F. Bonetti, R. Profeta, (Glaxo Group Ltd.), WO2007042240A1, 2007;
- 3d A. Hutchinson, B. Chenard, G. Li, M. Ghosh, J. Tarrant, T. Yoon, G. Luke, K. Lee, M. M. O′Donnell, W. Pringle, J. Peterson, K. Hodgetts, C. Steenstra, D. Doller, (Cantor Colburn LLP), US20060009456A1, 2006.
- 4
- 4aA. López, R. Pleixats, Tetrahedron: Asymmetry 1998, 9, 1967;
- 4bM. J. O’ Donnell, F. Delgado, E. Domìnguez, J. de Blas, W. L. Scott, Tetrahedron: Asymmetry 2001, 12, 821;
- 4cS. Arai, K. Tokumaru, T. Aoyama, Chem. Pharm. Bull. 2004, 52, 646;
- 4dB. Lygo, B. Allbutt, E. H. M. Kirton, Tetrahedron Lett. 2005, 46, 4461;
- 4eT. Ishikawa, Y. Araki, T. Kumamoto, H. Seki, K. Fukuda, T. Isobe, Chem. Commun. 2001, 245;
- 4fT. Akiyama, M. Hara, K. Fuchibe, S. Sakamoto, K. Yamaguchi, Chem. Commun. 2003, 1734;
- 4gE. J. Corey, M. C. Noe, F. Xu, Tetrahedron Lett. 1998, 39, 5347;
- 4hS. Saito, T. Tsubogo, S. Kobayashi, J. Am. Chem. Soc. 2007, 129, 5364;
- 4iS. Kobayashi, T. Tsubogo, S. Saito, Y. Yamashita, Org. Lett. 2008, 10, 807;
- 4jT. Tsubogo, S. Saito, K. Seki, Y. Yamashita, S. Kobayashi, J. Am. Chem. Soc. 2008, 130, 13321. For asymmetric additions of glycine derivatives to imines, see
- 4kX. X. Yan, Q. Peng, Q. Li, K. Zhang, J. Yao, X. L. Hou, Y. D. Wu, J. Am. Chem. Soc. 2008, 130, 14362. For SN2′ additions, see
- 4lC. G. Chen, X. L. Hou, L. Pu, Org. Lett. 2009, 11, 2073;
- 4mB. Lygo, C. Beynon, C. Lumley, M. C. McLeod, C. E. Wade, Tetrahedron Lett. 2009, 50, 3363;
- 4nT. Ma, X. Fu, C. W. Kee, L. Zong, Y. Pan, K. Huang, C. J. Tan, J. Am. Chem. Soc. 2011, 133, 2828;
- 4oX. Hou, Q. Li, J. Yao, L. Dai, Chin. J. Chem. 2010, 28, 1761.
- 5Efficient mixing of CsOH on a manufacturing scale is difficult because of its density. Also, waste disposal is more than 500-times more expensive when the waste contains cesium.
- 6Ketone 4 a is a bench-stable crystalline solid. See the Supporting Information for a preparative procedure.
- 7The ratios obtained by Kobayashi et al. for conjugate addition versus [3+2] cycloaddition were heavily dependent upon the structure of the substrate. To favor conjugate addition, the structure of 5 was modified (see Ref. [4i]), but for our purposes this is prohibitively expensive, and still requires 10 mol % of catalyst to proceed efficiently.
- 8All of these ligands were purchased from Solvias. For FOXAP ligands, see Y. Miyake, Y. Nishibayashi, S. Uemura, Synlett 2008, 1747, and references therein.
- 9See the Supporting Information for more details.
- 10
- 10aT. Osako, Y. Tachi, M. Doe, M. Shiro, K. Ohkubo, S. Fukuzumi, S. Itoh, Chem. Eur. J. 2004, 10, 237;
- 10bX. Ribas, R. Xifra, T. Parella, A. Poater, M. Solà, A. Llobet, Angew. Chem. 2006, 118, 3007;
10.1002/ange.200504222 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 2941.
- 11C. Díaz, Polyhedron 1997, 16, 999.
- 12Δ G=−RTlnK=−(0.001986 kcal mol−1 K−1)(298 K)ln (1/9)=−1.3.
- 13All calculation were performed with Gaussian 03 (Gaussian 03, revision D.02, M. J. Frisch et al.). See the Supporting Information.
- 14
- 14aX. Ye, Z.-H Li, W. Wang, K. Fan, W. Xu, Z. Hua, Chem. Phys. Lett. 2004, 397, 56;
- 14bX. X. Yan, Q. Peng, Y. Zhang, K. Zhang, W. Hong, X. L. Hou, Y. D. Wu, Angew. Chem. 2006, 118, 2013; Angew. Chem. Int. Ed. 2006, 45, 1979.