Nickel-Catalyzed [3+2+2] Cycloadditions between Alkynylidenecyclopropanes and Activated Alkenes†
Lucía Saya
Departamento de Química Orgánica,Centro Singular de Investigación en Química Biológica y Materiales Moleculares y Unidad Asociada al CSIC, Universidad de Santiago de Compostela, 15782, Santiago de Compostela (Spain), Fax: (+34) 981595-012
Search for more papers by this authorDr. Gaurav Bhargava
Departamento de Química Orgánica,Centro Singular de Investigación en Química Biológica y Materiales Moleculares y Unidad Asociada al CSIC, Universidad de Santiago de Compostela, 15782, Santiago de Compostela (Spain), Fax: (+34) 981595-012
Search for more papers by this authorMiguel A. Navarro
Departamento de Química Orgánica,Centro Singular de Investigación en Química Biológica y Materiales Moleculares y Unidad Asociada al CSIC, Universidad de Santiago de Compostela, 15782, Santiago de Compostela (Spain), Fax: (+34) 981595-012
Search for more papers by this authorDr. Moises Gulías
Departamento de Química Orgánica,Centro Singular de Investigación en Química Biológica y Materiales Moleculares y Unidad Asociada al CSIC, Universidad de Santiago de Compostela, 15782, Santiago de Compostela (Spain), Fax: (+34) 981595-012
Search for more papers by this authorCorresponding Author
Dr. Fernando López
Instituto de Química Orgánica General (CSIC), Juan de la Cierva 3, 28006, Madrid (Spain)
Fernando López, Instituto de Química Orgánica General (CSIC), Juan de la Cierva 3, 28006, Madrid (Spain)
José L. Mascareñas, Departamento de Química Orgánica,Centro Singular de Investigación en Química Biológica y Materiales Moleculares y Unidad Asociada al CSIC, Universidad de Santiago de Compostela, 15782, Santiago de Compostela (Spain), Fax: (+34) 981595-012
Search for more papers by this authorDr. Israel Fernández
Departamento de Química Orgánica, Universidad Complutense, Facultad de Ciencias Químicas, 28040 Madrid (Spain)
Search for more papers by this authorProf. Dr. Luis Castedo
Departamento de Química Orgánica,Centro Singular de Investigación en Química Biológica y Materiales Moleculares y Unidad Asociada al CSIC, Universidad de Santiago de Compostela, 15782, Santiago de Compostela (Spain), Fax: (+34) 981595-012
Search for more papers by this authorCorresponding Author
Prof. Dr. José L. Mascareñas
Departamento de Química Orgánica,Centro Singular de Investigación en Química Biológica y Materiales Moleculares y Unidad Asociada al CSIC, Universidad de Santiago de Compostela, 15782, Santiago de Compostela (Spain), Fax: (+34) 981595-012
Fernando López, Instituto de Química Orgánica General (CSIC), Juan de la Cierva 3, 28006, Madrid (Spain)
José L. Mascareñas, Departamento de Química Orgánica,Centro Singular de Investigación en Química Biológica y Materiales Moleculares y Unidad Asociada al CSIC, Universidad de Santiago de Compostela, 15782, Santiago de Compostela (Spain), Fax: (+34) 981595-012
Search for more papers by this authorLucía Saya
Departamento de Química Orgánica,Centro Singular de Investigación en Química Biológica y Materiales Moleculares y Unidad Asociada al CSIC, Universidad de Santiago de Compostela, 15782, Santiago de Compostela (Spain), Fax: (+34) 981595-012
Search for more papers by this authorDr. Gaurav Bhargava
Departamento de Química Orgánica,Centro Singular de Investigación en Química Biológica y Materiales Moleculares y Unidad Asociada al CSIC, Universidad de Santiago de Compostela, 15782, Santiago de Compostela (Spain), Fax: (+34) 981595-012
Search for more papers by this authorMiguel A. Navarro
Departamento de Química Orgánica,Centro Singular de Investigación en Química Biológica y Materiales Moleculares y Unidad Asociada al CSIC, Universidad de Santiago de Compostela, 15782, Santiago de Compostela (Spain), Fax: (+34) 981595-012
Search for more papers by this authorDr. Moises Gulías
Departamento de Química Orgánica,Centro Singular de Investigación en Química Biológica y Materiales Moleculares y Unidad Asociada al CSIC, Universidad de Santiago de Compostela, 15782, Santiago de Compostela (Spain), Fax: (+34) 981595-012
Search for more papers by this authorCorresponding Author
Dr. Fernando López
Instituto de Química Orgánica General (CSIC), Juan de la Cierva 3, 28006, Madrid (Spain)
Fernando López, Instituto de Química Orgánica General (CSIC), Juan de la Cierva 3, 28006, Madrid (Spain)
José L. Mascareñas, Departamento de Química Orgánica,Centro Singular de Investigación en Química Biológica y Materiales Moleculares y Unidad Asociada al CSIC, Universidad de Santiago de Compostela, 15782, Santiago de Compostela (Spain), Fax: (+34) 981595-012
Search for more papers by this authorDr. Israel Fernández
Departamento de Química Orgánica, Universidad Complutense, Facultad de Ciencias Químicas, 28040 Madrid (Spain)
Search for more papers by this authorProf. Dr. Luis Castedo
Departamento de Química Orgánica,Centro Singular de Investigación en Química Biológica y Materiales Moleculares y Unidad Asociada al CSIC, Universidad de Santiago de Compostela, 15782, Santiago de Compostela (Spain), Fax: (+34) 981595-012
Search for more papers by this authorCorresponding Author
Prof. Dr. José L. Mascareñas
Departamento de Química Orgánica,Centro Singular de Investigación en Química Biológica y Materiales Moleculares y Unidad Asociada al CSIC, Universidad de Santiago de Compostela, 15782, Santiago de Compostela (Spain), Fax: (+34) 981595-012
Fernando López, Instituto de Química Orgánica General (CSIC), Juan de la Cierva 3, 28006, Madrid (Spain)
José L. Mascareñas, Departamento de Química Orgánica,Centro Singular de Investigación en Química Biológica y Materiales Moleculares y Unidad Asociada al CSIC, Universidad de Santiago de Compostela, 15782, Santiago de Compostela (Spain), Fax: (+34) 981595-012
Search for more papers by this authorThis work was supported by the Spanish MEC [SAF2007-61015, Consolider-Ingenio 2010 (CSD2007-00006)], the CSIC, the Xunta de Galicia (GRC2010/12, INCITE09 209 122 PR, and PGIDIT06PXIB209126PR). We thank the Xunta de Galicia for an Isabel Barreto contract to L.S. and an Anxeles Alvariño contract to M.G. I.F. is a Ramon y Cajal fellow. We thank Johnson–Matthey for a gift of metals.
Graphical Abstract
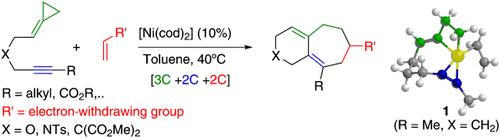
Now with nickel: [3C+2C+2C] cycloadditions involving non-activated alkylidenecyclopropanes provide a practical entry to a variety of interesting 6,7-fused bicyclic systems (see scheme; cod=1,5-cyclooctadiene). DFT calculations, combined with experimental data, suggest that the catalytic cycle involves the initial formation of 1-alkylidenenickelacyclobutane intermediates, such as 1.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201004438_sm_miscellaneous_information.pdf1.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. Rubin, M. Rubina, V. Gevorgyan, Chem. Rev. 2007, 107, 3117;
- 1bA. Brandi, S. Cicchi, F. M. Cordero, A. Goti, Chem. Rev. 2003, 103, 1213–1269;
- 1cM. Murakami, N. Ishida, T. Miura, Chem. Commun. 2006, 643–645;
- 1dR. Castro-Rodrigo, M. A. Esteruelas, S. Fuertes, A. M. López, F. López, J. L. Mascareñas, S. Mozo, E. Oñate, L. Saya, L. Villarino, J. Am. Chem. Soc. 2009, 131, 15572–15573;
- 1eR. Castro-Rodrigo, M. A. Esteruelas, A. M. López, F. López, J. L. Mascareñas, M. Oliván, E. Oñate, L. Saya, L. Villarino, J. Am. Chem. Soc. 2010, 132, 454–455.
- 2
- 2aA. Delgado, J. R. Rodríguez, L. Castedo, J. L. Mascareñas, J. Am. Chem. Soc. 2003, 125, 9282–9283;
- 2bJ. Durán, M. Gulías, L. Castedo, J. L. Mascareñas, Org. Lett. 2005, 7, 5693–5696;
- 2cM. Gulías, R. García, A. Delgado, L. Castedo, J. L. Mascareñas, J. Am. Chem. Soc. 2006, 128, 384–385;
- 2dB. Trillo, M. Gulías, F. López, L. Castedo, J. L. Mascareñas, Adv. Synth. Catal. 2006, 348, 2381–2384;
- 2eM. Gulías, J. Durán, F. López, L. Castedo, J. L. Mascareñas, J. Am. Chem. Soc. 2007, 129, 11026–11027;
- 2fR. García-Fandiño, M. Gulías, L. Castedo, J. R. Granja, J. L. Mascareñas, D. J. Cárdenas, Chem. Eur. J. 2008, 14, 272—281; For a ruthenium-catalyzed example, see:
- 2gF. López, A. Delgado, J. R. Rodríguez, L. Castedo, J. L. Mascareñas, J. Am. Chem. Soc. 2004, 126, 10262–10263.
- 3G. Bhargava, B. Trillo, M. Araya, F. López, L. Castedo, J. L. Mascareñas, Chem. Commun. 2010, 46, 270–272.
- 4P. A. Evans, P. A. Inglesby, J. Am. Chem. Soc. 2008, 130, 12838–12839.
- 5For a review, see: P. Binger, H. M. Buch, Top. Curr. Chem. 1987, 135, 77–151.
- 6Alkylidenecyclopropanes usually participate in nickel-catalyzed intermolecular [3+2] cycloadditions by cleavage of the distal carbon bond of the cyclopropane ring. However, the cycloadditions between methylenecyclopropane and certain activated alkenes, in the presence of “naked” nickel catalysts, provide adducts arising from the cleavage of the proximal bond. For examples, see:
- 6aR. Noyori, T. Odagi, H. Takaya, J. Am. Chem. Soc. 1970, 92, 5780–5781;
- 6bR. Noyori, Y. Kumagai, I. Umeda, H. Takaya, J. Am. Chem. Soc. 1972, 94, 4018–4020;
- 6cP. Binger, Angew. Chem. Int. Ed. Eng. 1972, 11, 309–310;
- 6dP. Binger, Synthesis 1973, 427–428;
- 6eP. Binger, J. Mcmeekin, Angew. Chem. 1973, 85, 1053–1054; Angew. Chem. Int. Ed. Engl. 1973, 12, 995–996;
- 6fP. Binger, E. Sternberg, U. Wittig, Chem. Ber. 1987, 120, 1933–1938;
- 6gR. Noyori, M. Yamakawa, Tetrahedron Lett. 1978, 19, 4823–4826;
10.1016/S0040-4039(01)85742-7 Google Scholar
- 6hP. Binger, A. Brinkmann, W. J. Richter, Tetrahedron Lett. 1983, 24, 3599–3602;
- 6iP. Binger, P. Wedemann, Tetrahedron Lett. 1983, 24, 5847–5850;
- 6jP. Binger, P. Wedemann, Tetrahedron Lett. 1985, 26, 1045–1048;
- 6kT. Kawasaki, S. Saito, Y. Yamamoto, J. Org. Chem. 2002, 67, 4911–4915.
- 7Saito and co-workers have developed a series of nickel-catalyzed intermolecular [3+2+2] cycloadditions involving proximal-bond cleavage of the cyclopropane ring. However, these methods are limited to the use of alkynes as two-carbon components and cyclopropylideneacetates as three-carbon partners, as the presence of the ester group on the ACP is mandatory for the cycloaddition. For examples, see:
- 7aS. Saito, M. Masuda, S. Komagawa, J. Am. Chem. Soc. 2004, 126, 10540–10541;
- 7bS. Komagawa, S. Saito, Angew. Chem. 2006, 118, 2506–2509;
10.1002/ange.200504050 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 2446–2449;
- 7cS. Saito, S. Komagawa, I. Azumaya, M. Masuda, J. Org. Chem. 2007, 72, 9114–9120;
- 7dR. Yamasaki, N. Terashima, I. Sotome, S. Komagawa, S. Saito, J. Org. Chem. 2010, 75, 480–483, and references therein.
- 8For a nickel-catalyzed [3+2+2] cycloaddition using bicyclopropylidene (3C) involving a proximal-bond cleavage, see: L. Zhao, A. de Meijere, Adv. Synth. Catal. 2006, 348, 2484–2492.
- 9Increasing the temperature (up to 90 °C), modifying the concentration of 1 a, or increasing the number of equivalents of methyl vinyl ketone did not provide any substantial improvement in the yield or selectivity.
- 10For further details, see the Supporting Information.
- 11Similarly, alkenes with substituents at their β position, such as ethyl 2-butenoate, failed to participate in the cycloaddition reaction.
- 12Slow addition (5 h) of the reactants (1 b and MVK) to a solution of [Ni(cod)2] (20 mol %) did not improve the conversion.
- 13Performing the cycloaddition between 1 b and MVK (10 equiv) in the presence of 20 mol % of cycloadduct 5 ba afforded 30 % conversion. Increasing the amount of 5 ba to up to 90 % led to an even lower conversion (18 %). Comparison of these results with those of Table 3 (entry 1) suggests that the cycloadduct 5 ba inhibits the cycloaddition, probably by coordinating to the nickel catalyst.
- 14Substrates with terminal alkyne groups led to recovery of the majority of the starting material.
- 15CCDC 791310 (5 ga) and 782973 (5 fa) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 16All the calculations were carried out at the B3LYP/def2-SVP level using the Gaussian 03 rev. D.01 suite of programs. For computational details, see the Supporting Information.
- 17Nickelacylobutane E-i could be involved in the formation of the [3+2] cycloaddition products of type 6, as the alkene moiety of these cycloadducts exhibits E stereochemistry. However, the requirements of the intramolecularly linked alkyne (Scheme 3) suggests that alternative mechanisms, probably involving alkyne-stabilized nickelacyclopentenes, might be operating.[7] It is possible that the higher selectivity observed for 1 d in comparison to 1 c (Table 2, entries 5 and 6) might be related to a greater difficulty in producing such intermediates owing to steric reasons.
- 18Despite extensive investigation, a transition state corresponding to a reductive elimination from iiia or iiib, to afford an intramolecular [3+2] cycloadduct, could not be located computationally. This result is in agreement with experimental results, as these types of 6,5-bicyclic systems were never detected.
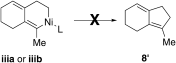
- 19DFT calculations on a substrate bearing an ester group instead of the methyl group at the alkyne moiety revealed lower energy barriers for the migratory-insertion steps eventually leading to the [3+2+2] adducts. This result is consistent with the higher [3+2+2]/[3+2] selectivity observed experimentally for this type of precursor (1 b).
- 20Regioisomers of type 9 were never observed. In agreement with this results, DFT calculations showed that the corresponding pathway leading to these products is more energetic than that affording 5.