Analysis and Optimization of Copper-Catalyzed Azide–Alkyne Cycloaddition for Bioconjugation†
Vu Hong
Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA), Fax: (+1) 858-784-8850
Search for more papers by this authorStanislav I. Presolski
Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA), Fax: (+1) 858-784-8850
Search for more papers by this authorCelia Ma
Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA), Fax: (+1) 858-784-8850
Search for more papers by this authorM. G. Finn Prof.
Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA), Fax: (+1) 858-784-8850
Search for more papers by this authorVu Hong
Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA), Fax: (+1) 858-784-8850
Search for more papers by this authorStanislav I. Presolski
Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA), Fax: (+1) 858-784-8850
Search for more papers by this authorCelia Ma
Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA), Fax: (+1) 858-784-8850
Search for more papers by this authorM. G. Finn Prof.
Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA), Fax: (+1) 858-784-8850
Search for more papers by this authorThis work was supported by The Skaggs Institue for Chemical Biology, Pfizer, Inc., and the NIH (RR021886). We are grateful to Matthias Park for assistance with the synthesis of 3.
Graphical Abstract
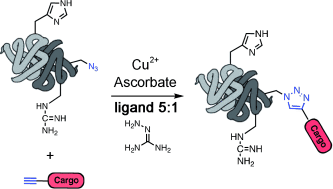
How to click with biomolecules: Copper-catalyzed azide–alkyne cycloaddition has been optimized for use with biological molecules. The key development is the addition of two reagents that allow ascorbate to be used as reducing agent whilst eliminating problems caused by copper ascorbate side reactions. The result is a robust, rapid, and convenient procedure for the modification of proteins, DNA, RNA, and other biomolecules (see scheme).
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200905087_sm_miscellaneous_information.pdf1.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aC. W. Tornøe, C. Christensen, M. Meldal, J. Org. Chem. 2002, 67, 3057;
- 1bV. V. Rostovtsev, L. G. Green, V. V. Fokin, K. B. Sharpless, Angew. Chem. 2002, 114, 2708;
10.1002/1521-3757(20020715)114:14<2708::AID-ANGE2708>3.0.CO;2-0 Google ScholarAngew. Chem. Int. Ed. 2002, 41, 2596.10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4 CAS PubMed Web of Science® Google Scholar
- 2H. C. Kolb, M. G. Finn, K. B. Sharpless, Angew. Chem. 2001, 113, 2056;
Angew. Chem. Int. Ed. 2001, 40, 2004.
10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5 CAS PubMed Web of Science® Google Scholar
- 3
- 3aM. D. Best, Biochemistry 2009, 48, 6571;
- 3bW. H. Binder, R. Sachsenhofer, Macromol. Rapid Commun. 2008, 29, 952;
- 3cA. D. Moorhouse, J. E. Moses, ChemMedChem 2008, 3, 715;
- 3dH. Nandivada, X. Jiang, J. Lahann, Adv. Mater. 2007, 19, 2197;
- 3eH. C. Kolb, K. B. Sharpless, Drug Discovery Today 2003, 8, 1128.
- 4
- 4aN. J. Agard, J. A. Prescher, C. R. Bertozzi, J. Am. Chem. Soc. 2004, 126, 15046;
- 4bJ. M. Baskin, J. A. Prescher, S. T. Laughlin, N. J. Agard, P. V. Chang, I. A. Miller, A. Lo, J. A. Codelli, C. R. Bertozzi, Proc. Natl. Acad. Sci. USA 2007, 104, 16793;
- 4cJ. A. Codelli, J. M. Baskin, N. J. Agard, C. R. Bertozzi, J. Am. Chem. Soc. 2008, 130, 11486.
- 5
- 5aA. E. Speers, B. F. Cravatt, Chem. Biol. 2004, 11, 535;
- 5bK. E. Beatty, F. Xie, Q. Wang, D. A. Tirrell, J. Am. Chem. Soc. 2005, 127, 14150;
- 5cJ. Gierlich, K. Gutsmiedl, P. M. E. Gramlich, A. Schmidt, G. A. Burley, T. Carell, Chem. Eur. J. 2007, 13, 9486;
- 5dT.-L. Hsu, S. R. Hanson, K. Kishikawa, S.-K. Wang, M. Sawa, C.-H. Wong, Proc. Natl. Acad. Sci. USA 2007, 104, 2614.
- 6
- 6aM. R. Levengood, C. C. Kerwood, C. Chatterjee, W. A. van der Donk, ChemBioChem 2009, 10, 911;
- 6bM. Galibert, P. Dumy, D. Boturyn, Angew. Chem. 2009, 121, 2614;
10.1002/ange.200806223 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 2576.
- 7
- 7aT. R. Chan, R. Hilgraf, K. B. Sharpless, V. V. Fokin, Org. Lett. 2004, 6, 2853;
- 7bV. O. Rodionov, S. I. Presolski, S. Gardinier, Y. H. Lim, M. G. Finn, J. Am. Chem. Soc. 2007, 129, 12696.
- 8W. G. Lewis, F. G. Magallon, V. V. Fokin, M. G. Finn, J. Am. Chem. Soc. 2004, 126, 9152.
- 9S. Sen Gupta, J. Kuzelka, P. Singh, W. G. Lewis, M. Manchester, M. G. Finn, Bioconjugate Chem. 2005, 16, 1572.
- 10V. Hong, A. K. Udit, R. A. Evans, M. G. Finn, ChemBioChem 2008, 9, 1481.
- 11
- 11aA. J. Link, D. A. Tirrell, J. Am. Chem. Soc. 2003, 125, 11164;
- 11bD. C. Dieterich, J. J. Lee, A. J. Link, J. Graumann, D. A. Tirrell, E. M. Schuman, Nat. Protoc. 2007, 2, 532.
- 12
- 12aS. I. van Kasteren, H. B. Kramer, H. H. Jensen, S. J. Campbell, J. Kirkpatrick, N. J. Oldham, D. C. Anthony, B. G. Davis, Nature 2007, 446, 1105;
- 12bS. I. Van Kasteren, H. B. Kramer, D. P. Gamblin, B. G. Davis, Nat. Protoc. 2007, 2, 3185.
- 13
- 13aM. A. Bruckman, G. Kaur, L. A. Lee, F. Xie, J. Sepulvecla, R. Breitenkamp, X. Zhang, M. Joralemon, T. P. Russell, T. Emrick, Q. Wang, ChemBioChem 2008, 9, 519;
- 13bR. Kumar, A. El-Sagheer, J. Tumpane, P. Lincoln, L. M. Wilhelmsson, T. Brown, J. Am. Chem. Soc. 2007, 129, 6859;
- 13cP. Kocalka, A. H. El-Sagheer, T. Brown, ChemBioChem 2008, 9, 1280;
- 13dA. H. Ei-Sagheer, T. Brown, J. Am. Chem. Soc. 2009, 131, 3958;
- 13eA. J. Dirks, J. J. L. M. Cornelissen, R. J. M. Nolte, Bioconjugate Chem. 2009, 20, 1129.
- 14
- 14aQ. Wang, T. R. Chan, R. Hilgraf, V. V. Fokin, K. B. Sharpless, M. G. Finn, J. Am. Chem. Soc. 2003, 125, 3192;
- 14bS. C. Fry, Biochem. J. 1998, 332, 507.
- 15E. Lallana, E. Fernandez-Megia, R. Riguera, J. Am. Chem. Soc. 2009, 131, 5748.
- 16P. Y. Liu, N. Jiang, J. Zhang, X. Wei, H. H. Lin, X. Q. Yu, Chem. Biodiversity 2006, 3, 958.
- 17R. H. Nagaraj, D. R. Sell, M. Prabhakaram, B. J. Ortwerth, V. M. Monnier, Proc. Natl. Acad. Sci. USA 1991, 88, 10257.
- 18K. Sivakumar, F. Xie, B. M. Cash, S. Long, H. N. Barnhill, Q. Wang, Org. Lett. 2004, 6, 4603.
- 19S. I. Presolski, V. Hong, M. G. Finn, 2009, unpublished results.
- 20V. O. Rodionov, S. I. Presolski, D. D. Díaz, V. V. Fokin, M. G. Finn, J. Am. Chem. Soc. 2007, 129, 12705.
- 21W. L. Baker, J. Goode, L. Cooper, Mikrochim. Acta 1992, 106, 143.
- 22
- 22aY. Liu, G. Sun, A. David, L. M. Sayre, Chem. Res. Toxicol. 2004, 17, 110;
- 22bK. Uchida, S. Kawakishi, Biochem. Biophys. Res. Commun. 1986, 138, 659.
- 23A more polar compound derived from the ligand was observed but not characterized during these experiments. This material is presumably produced by oxidation at one of the CH sites adjacent to the triazole ring, or at the central nitrogen atom.
- 24N. Shangari, T. S. Chan, K. Chan, S. H. Wu, P. J. O’Brien, Mol. Nutr. Food Res. 2007, 51, 445.
- 25P. J. Thornalley, Chem. Biol. Interact. 1998, 111–112, 137.
- 26
- 26aE. Strable, D. E. Prasuhn, A. K. Udit, S. Brown, A. J. Link, J. T. Ngo, G. Lander, J. Quispe, C. S. Potter, B. Carragher, D. A. Tirrell, M. G. Finn, Bioconjugate Chem. 2008, 19, 866;
- 26bE. Kaltgrad, M. K. O’Reilly, L. Liao, S. Han, J. Paulson, M. G. Finn, J. Am. Chem. Soc. 2008, 130, 4578;
- 26cD. E. Prasuhn, P. Singh, E. Strable, S. Brown, M. Manchester, M. G. Finn, J. Am. Chem. Soc. 2008, 130, 1328.
- 27V. Hong, A. A. Kislukhin, M. G. Finn, J. Am. Chem. Soc. 2009, 131, 9986.
- 28
- 28aR. Agarwal, M. N. Gupta, Biotechnol. Tech. 1994, 8, 655;
- 28bT. B. Osborne, C. S. Leavenworth, J. Biol. Chem. 1916, 28, 109.