Stepwise Oxygenation of Pinacolborane by a Rhodiumperoxo Complex: Detection of an Intermediate Metal Borate and Perborate†
Marcel Ahijado Salomon Dr.
Institut für Chemie, Humboldt-Universität zu Berlin, Brook-Taylor-Strasse 2, 12489 Berlin (Germany), Fax: (+49) 30-2093-6939
Search for more papers by this authorThomas Braun Prof. Dr.
Institut für Chemie, Humboldt-Universität zu Berlin, Brook-Taylor-Strasse 2, 12489 Berlin (Germany), Fax: (+49) 30-2093-6939
Search for more papers by this authorAnna Penner Dipl.-Chem.
Institut für Chemie, Humboldt-Universität zu Berlin, Brook-Taylor-Strasse 2, 12489 Berlin (Germany), Fax: (+49) 30-2093-6939
Search for more papers by this authorMarcel Ahijado Salomon Dr.
Institut für Chemie, Humboldt-Universität zu Berlin, Brook-Taylor-Strasse 2, 12489 Berlin (Germany), Fax: (+49) 30-2093-6939
Search for more papers by this authorThomas Braun Prof. Dr.
Institut für Chemie, Humboldt-Universität zu Berlin, Brook-Taylor-Strasse 2, 12489 Berlin (Germany), Fax: (+49) 30-2093-6939
Search for more papers by this authorAnna Penner Dipl.-Chem.
Institut für Chemie, Humboldt-Universität zu Berlin, Brook-Taylor-Strasse 2, 12489 Berlin (Germany), Fax: (+49) 30-2093-6939
Search for more papers by this authorWe would like to acknowledge the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie for financial support.
Graphical Abstract
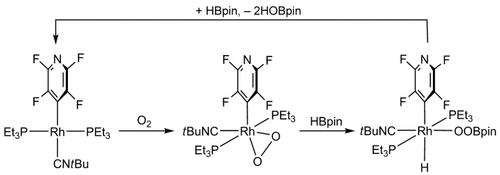
Acting as go-between: A rhodium borate and perborate have been identified as intermediates in the rhodium-mediated oxygenation of pinacolborane (HBpin; see scheme). The rhodium(III)–peroxo complex reacts with the Lewis acidic boron compound HBpin by oxygen transfer from the rhodium center to HBpin to give a rhodium(I) species. The reaction sequence might play a crucial role in the homocoupling of boronic acid.
References
- 1See for example:
- 1aR. A. Sheldon, J. K. Kochi, Metal-Catalyzed Oxidations of Organic Compounds, Academic Press, New York, 1981;
- 1bC. Limberg, Angew. Chem. 2003, 115, 6112–6136;
10.1002/ange.200300578 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 5932–5954;
- 1c Topics in Organometallic Chemistry (Eds.: ), Springer, Berlin, 2007;
- 1dJ.-M. Brégeault, Dalton Trans. 2003, 3289–3302;
- 1e Modern Oxidation Methods (Ed.: ), Wiley-VCH, Weinheim, 2004.
- 2aM. Krom, T. P. J. Peters, R. G. E. Coumans, T. J. J. Sciarone, J. Hoogboom, S. I. ter Beek, P. J. P. Schlebos, J. M. M. Smits, R. de Gelder, A. W. Gal, Eur. J. Inorg. Chem. 2003, 1072–1087;
- 2bM. P. del Río, M. A. Ciriano, C. Tejel, Angew. Chem. 2008, 120, 2536–2539;
10.1002/ange.200705802 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 2502–2505;
- 2cC. Tejel, M. A. Ciriano, S. Jiménez, V. Passarelli, J. A. López, Angew. Chem. 2008, 120, 2123–2126;
10.1002/ange.200702559 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 2093–2096;
- 2dT. Nishimura, N. Kakiuchi, T. Onoue, K. Ohe, S. Uemura, J. Chem. Soc. Perkin Trans. 1 2000, 1915–1918;
- 2eF. Igersheim, H. Mimoun, Nouv. J. Chim. 1980, 4, 711–713;
- 2fM. Krom, R. G. E. Coumans, J. M. M. Smits, A. W. Gal, Angew. Chem. 2002, 114, 595–599;
10.1002/1521-3757(20020215)114:4<595::AID-ANGE595>3.0.CO;2-R Google ScholarAngew. Chem. Int. Ed. 2002, 41, 575–579;
- 2gM. Krom, R. G. E. Coumans, J. M. M. Smits, A. W. Gal, Angew. Chem. 2001, 113, 2164–2166;
10.1002/1521-3757(20010601)113:11<2164::AID-ANGE2164>3.0.CO;2-# Google ScholarAngew. Chem. Int. Ed. 2001, 40, 2106–2108;10.1002/1521-3773(20010601)40:11<2106::AID-ANIE2106>3.0.CO;2-O CAS PubMed Web of Science® Google Scholar
- 2hB. de Bruin, P. H. M. Budzelaar, A. W. Gal, Angew. Chem. 2004, 116, 4236–4251; Angew. Chem. Int. Ed. 2004, 43, 4142–4157.
- 3
- 3aN. R. Conley, L. A. Labios, D. M. Pearson, C. C. L. McCroy, R. M. Waymouth, Organometallics 2007, 26, 5447–5453;
- 3bS. S. Stahl, Angew. Chem. 2004, 116, 3480–3501;
10.1002/ange.200300630 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 3400–3420;
- 3cB. A. Steinhoff, I. A. Guzei, S. S. Stahl, J. Am. Chem. Soc. 2004, 126, 11268–11278;
- 3dD. R. Jensen, M. J. Schultz, J. A. Mueller, M. S. Sigman, Angew. Chem. 2003, 115, 3940–3943; Angew. Chem. Int. Ed. 2003, 42, 3810–3813;
- 3eM. M. Konnick, B. A. Gandhi, I. A. Guzei, S. S. Stahl, Angew. Chem. 2006, 118, 2970–2973;
10.1002/ange.200600532 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 2904–2907;
- 3fB. A. Steinhoff, S. R. Fix, S. S. Stahl, J. Am. Chem. Soc. 2002, 124, 766–767;
- 3gG.-J. ten Brink, I. W. C. E. Arends, R. A. Sheldon, Adv. Synth. Catal. 2002, 344, 355–369;
- 3hJ. A. Mueller, C. P. Goller, M. S. Sigman, J. Am. Chem. Soc. 2004, 126, 9724–9734;
- 3iJ. M. Keith, R. J. Nielsen, J. Oxgaard, W. A. Goddard III, J. Am. Chem. Soc. 2005, 127, 13172–13179;
- 3jC. N. Cornell, M. S. Sigman, Inorg. Chem. 2007, 46, 1903–1909;
- 3kK. M. Gligorich, M. S. Sigman, Angew. Chem. 2006, 118, 6764–6767;
10.1002/ange.200602138 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 6612–6615;
- 3lJ. Piera, J.-E. Bäckvall, Angew. Chem. 2008, 120, 3558–3576;
10.1002/ange.200700604 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 3506–3523;
- 3mM. M. Konnick, S. S. Stahl, J. Am. Chem. Soc. 2008, 130, 5753–5762.
- 4C. Adamo, C. Amatore, I. Ciofini, A. Jutand, H. Lakmini, J. Am. Chem. Soc. 2006, 128, 6829–6836.
- 5
- 5aM. A. Aramendia, F. Lafont, M. Moreno-Mañas, R. Pleixats, A. Roglans, J. Org. Chem. 1999, 64, 3592–3594;
- 5bK. A. Smith, E. M. Campi, W. R. Jackson, S. Marcuccio, C. G. M. Naeslund, G. B. Deacon, Synlett 1997, 131–132;
- 5cJ. P. Parrish, Y. C. Jung, R. J. Floyd, K. W. Jung, Tetrahedron Lett. 2002, 43, 7899–7902;
- 5dH. Yoshida, Y. Yamaryo, J. Ohshita, A. Kunai, Tetrahedron Lett. 2003, 44, 1541–1544.
- 6Z. Heiden, T. B. Rauchfuss, J. Am. Chem. Soc. 2007, 129, 14303–14310, and references therein.
- 7
- 7aT. M. Boller, J. M. Murphy, M. Hapke, T. Ishiyama, N. Miyaura, J. F. Hartwig, J. Am. Chem. Soc. 2005, 127, 14263–14278;
- 7bT. Ishiyama, J. Takagi, K. Ishida, N. Miyaura, N. R. Anastasi, J. F. Hartwig, J. Am. Chem. Soc. 2002, 124, 390–391;
- 7cT. Ishiyama, Y. Nobuta, J. F. Hartwig, Chem. Commun. 2003, 2924–2925;
- 7dW. F. Lo, H. M. Kaiser, A. Spannenberg, M. Beller, M. K. Tse, Tetrahedron Lett. 2007, 48, 371–375;
- 7eK. Kawamura, J. F. Hartwig, J. Am. Chem. Soc. 2001, 123, 8422–8423;
- 7fC. C. Tzschucke, J. M. Murphy, Org. Lett. 2007, 9, 761–764;
- 7gJ.-Y. Cho, C. N. Iverson, M. R. Smith III, J. Am. Chem. Soc. 2000, 122, 12868–12869;
- 7hJ.-Y. Cho, M. K. Tse, D. Holmes, R. E. Maleczka, Jr., M. R. Smith III, Science 2002, 295, 305–308.
- 8
- 8aS. Shimada, A. S. Batsanov, J. A. K. Howard, T. B. Marder, Angew. Chem. 2001, 113, 2226–2229;
10.1002/1521-3757(20010601)113:11<2226::AID-ANGE2226>3.0.CO;2-Q Google ScholarAngew. Chem. Int. Ed. 2001, 40, 2168–2171;10.1002/1521-3773(20010601)40:11<2168::AID-ANIE2168>3.0.CO;2-0 CAS PubMed Web of Science® Google Scholar
- 8bW. H. Lam, K. C. Lam, Z. Lin, S. Shimada, R. N. Perutz, T. B. Marder, Dalton Trans. 2004, 1556–1562;
- 8cR. J. Lindup, T. B. Marder, R. N. Perutz, A. C. Whitwood, Chem. Commun. 2007, 3664–3666.
- 9
- 9aK. Burgess, W. A. van der Donk, S. A. Westcott, T. B. Marder, R. T. Baker, J. C. Calabrese, J. Am. Chem. Soc. 1992, 114, 9350–9359;
- 9bR. B. Coapes, F. E. S. Souza, R. L. Thomas, J. J. Hall, T. B. Marder, Chem. Commun. 2003, 613–615;
- 9cV. J. Olsson, K. J. Szabó, Angew. Chem. 2007, 119, 7015–7017;
10.1002/ange.200702499 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 6891–6893;
- 9dD. I. McIsaac, S. J. Geier, C. M. Vogels, A. Decken, S. A. Wetcott, Inorg. Chim. Acta 2006, 359, 2771–2779;
- 9eI. A. I. Mkhalid, R. B. Coapes, S. N. Edes, D. N. Coventry, F. E. S. Souza, R. L. Thomas, J. J. Hall, S.-W. Bi, Z. Lin, T. B. Marder, Dalton Trans. 2007, 1055–1064;
- 9fC. B. Fritschi, S. M. Wernitz, C. M. Vogels, M. P. Shaver, A. Decken, A. Bell, S. A. Westcott, Eur. J. Inorg. Chem. 2008, 779–785;
- 9gH. Braunschweig, Angew. Chem. 1998, 110, 1882–1898;
10.1002/(SICI)1521-3757(19980703)110:13/14<1882::AID-ANGE1882>3.0.CO;2-5 Web of Science® Google ScholarAngew. Chem. Int. Ed. 1998, 37, 1786–1801;10.1002/(SICI)1521-3773(19980803)37:13/14<1786::AID-ANIE1786>3.0.CO;2-C CAS Web of Science® Google Scholar
- 9hH. Braunschweig, M. Colling, Coord. Chem. Rev. 2001, 223, 1–51;
- 9iG. J. Irvine, M. J. G. Lesley, T. B. Marder, N. C. Norman, C. R. Rice, E. G. Robins, W. R. Roper, G. R. Whittell, J. L. Wright, Chem. Rev. 1998, 98, 2685–2722.
- 10
- 10aJ. F. Hartwig, K. S. Cook, M. Hapke, C. D. Incarvito, Y. Fan, C. E. Webster, M. B. Hall, J. Am. Chem. Soc. 2005, 127, 2538–2552;
- 10bH. Chen, S. Schlecht, T. C. Semple, J. F. Hartwig, Science 2000, 287, 1995–1997.
- 11
- 11aM. Ahijado, T. Braun, D. Noveski, N. Kocher, B. Neumann, D. Stalke, H.-G. Stammler, Angew. Chem. 2005, 117, 7107–7111;
10.1002/ange.200501615 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 6947–6951;
- 11bM. Ahijado, T. Braun, Angew. Chem. 2008, 120, 2996–3000;
10.1002/ange.200705715 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 2954–2958.
- 12
- 12aJ. K. Kochi, M. J. Y. Chen, J. Chem. Soc. Chem. Commun. 1977, 204–205;
- 12bC. W. Dudley, G. Read, P. J. C. Walker, J. Chem. Soc. Dalton Trans. 1974, 1926–1931.
- 13D. Noveski, T. Braun, B. Neumann, A. Stammler, H.-G. Stammler, Dalton Trans. 2004, 4106–4119.
- 14
- 14aR. Pizer, C. Tihal, Inorg. Chem. 1987, 26, 3639–3642;
- 14bD. M. Davies, M. E. Deary, K. Quill, R. A. Smith, Chem. Eur. J. 2005, 11, 3552–3558;
- 14cY. A. Alexandrov, N. V. Chikinova, J. Organomet. Chem. 1991, 418, 1–59.
- 15H. Hermans, Z. Anorg. Allg. Chem. 1925, 142, 83–110.
- 16T. Braun, D. Noveski, M. Ahijado, F. Wehmeier, Dalton Trans. 2007, 3820–3825.
- 17V. P. Maslennikov, G. I. Makin, V. N. Alyaov, Y. A. Aleksandrov, Zh. Obshch. Khim. 1973, 43, 1972–1973.
- 18P. Zhao, C. D. Incarvito, J. F. Hartwig, J. Am. Chem. Soc. 2007, 129, 1876–1877.
- 19D. S. Laitar, P. Müller, J. P. Sadighi, J. Am. Chem. Soc. 2005, 127, 17196–17197.
- 20S. Hawkeswood, D. W. Stephan, Dalton Trans. 2005, 2182–2187.
- 21Data for the X-ray structure analysis of 3: C28H45BF4N2O3P2Rh, Mr=709.32, crystal dimensions 0.15×0.07×0.03 mm3; monoclinic; P21/c; a=10.3187(5), b=35.2256(13), c=10.0646(5) Å, β=110.548(4), Z=4, V=3425.6(3) Å3, ρcalcd=1.375 g cm−3; 2θmax=50.0°, MoKα radiation (λ=0.71073 Å), T=100(2) K, 25 645 reflections collected, 5950 were unique (Rint.=0.1331), STOE IPDS 2Θ diffractometer, numerical absorption correction (min./max. transmission 0.910/0.981), μ=0.643 mm−1. The structure was solved by direct methods and refined with full-matrix least-square methods on F2 (SHELX-97).[30] Final R1, wR2 values on all data: 0.1486, 0.1149; R1, wR2 values for 5950 reflections with I0>2σ(I0): 0.0695, 0.0982, residual electron density +1.101/−0.486 e Å−3, hydrogen atoms were placed at calculated positions and refined using a riding model, the disordered pinacole unit was refined isotropically on two positions (58:42), the disordered CH2 groups (C17, C21) were refined on two positions (57:43 and 76:24), the corresponding ethyl groups were restrained with “sadi” instructions and were refined isotropically. CCDC 694588 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 22L. H. Doerrer, J. R. Galsworthy, M. L. H. Green, M. A. Leech, M. Müller, J. Chem. Soc. Dalton Trans. 1998, 3191–3194.
- 23
- 23aM. M. Konnick, I. A. Guzei, S. S. Stahl, J. Am. Chem. Soc. 2004, 126, 10212–10213;
- 23bJ. T. York, A. Llobet, C. J. Cramer, W. B. Tolman, J. Am. Chem. Soc. 2007, 129, 7990–7999.
- 24
- 24aM. Aresta, I. Tommasi, E. Quaranta, C. Fragale, J. Mascetti, M. Tranquille, F. Galan, M. Fouassier, Inorg. Chem. 1996, 35, 4254–4260.
- 25
- 25aM. Aresta, A. Dibenedetto, I. Tommasi, Eur. J. Inorg. Chem. 2001, 1801–1806;
- 25bT. Braun, A. Steffen, V. Schorlemer, B. Neumann, H.-G. Stammler, Dalton Trans. 2005, 3331–3336;
- 25cT. Braun, V. Schorlemer, B. Neumann, H.-G. Stammler, J. Fluorine Chem. 2006, 127, 367–372;
- 25dT. Braun, S. Rothfeld, A. Stammler, H.-G. Stammler, Inorg. Chem. Commun. 2003, 6, 752–755.
- 26
- 26aO. Blum, D. Milstein, Angew. Chem. 1995, 107, 210–212;
10.1002/ange.19951070214 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 229–231;
- 26bT. Yoshida, T. Matsuda, T. Okano, T. Kitani, S. Otsuka, J. Am. Chem. Soc. 1979, 101, 2027–2038;
- 26cD. S. Glueck, L. J. Newman, Winslow, R. G. Bergman, Organometallics 1991, 10, 1462–1479;
- 26dD. Milstein, J. C. Calabrese, I. D. Williams, J. Am. Chem. Soc. 1986, 108, 6387–6389.
- 27
- 27aT. Braun, J. Izundu, A. Steffen, B. Neumann, H.-G. Stammler, Dalton Trans. 2006, 5118–5123;
- 27bA. Steffen, T. Braun, B. Neumann, H.-G. Stammler, Angew. Chem. 2007, 119, 8828–8832;
10.1002/ange.200703393 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8674–8678;
- 27cT. Braun, B. Blöcker, V. Schorlemer, B. Neumann, A. Stammler, H.-G. Stammler, J. Chem. Soc. Dalton Trans. 2002, 2213–2218;
- 27dT. Braun, M. I. Sladek, B. Neumann, H.-G. Stammler, New J. Chem. 2003, 27, 313–318.
- 28
- 28aK. L. Fujdala, A. G. Oliver, F. J. Hollander, T. Don Tilley, Inorg. Chem. 2003, 42, 1140–1150;
- 28bA. Dibas, J. Howard, S. Anwar, D. Stewart, A. Khan, Biochem. Biophys. Res. Commun. 2000, 270, 383–386.
- 29Note that rhodium complexes have recently been described which bind oxygen with no net change in the oxidation state of the metal: J. M. Praetorius, D. P. Allen, R. Wang, J. D. Webb, F. Grein, P. Kennepohl, C. M. Crudden, J. Am. Chem. Soc. 2008, 130, 3724–3725.
- 30G. M. Sheldrick, SHELXS-97, Program for Crystal Structure Solution; SHELX-97, Program for Crystal Structure Refinement, University of Göttingen 1997.