[12]Annulene Gemini Surfactants: Structure and Self-Assembly
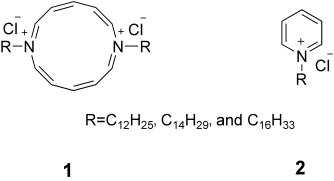
The authors of this Communication wish to alter the proposed structure of annulene 1, a system that had previously been reported by Yamaguchi et al.1 After one of the authors (F.M.M.) had originally inspected the then available analytical data (1H and 13C NMR spectra, elemental analysis, and ESI-MS data), he affirmed that they were (and still are) consistent with the annulene system. In particular, parent mass spectral signals at m/z 531.44353, 587.50648, and 643.56867 for the R=C12H25, C14H29, and C16H33 derivatives, respectively, all correspond to the mass of [1−Cl−]+. Recently, however, Prof. M. Cristl suggested2 that the pyridinium salt 2 would be an alternative and more likely possibility. There exists an intriguing ambiguity in this case because 1 and 2 have indistinguishable NMR spectra and elemental analyses and because our [1−2 Cl−]2+ base peak and the [2−Cl−]+ parent peak happen to have identical masses. We are now able to differentiate the two structures through weak 13C-containing MS signals. These signals have a shift one mass unit higher than the all-12C signal (consistent with [2−Cl−]+) as opposed to 0.5 units higher (consistent with [1−2 Cl−]2+). In view of these new data, our peaks at m/z>500 must, we surmise, arise from dimers of 2 in the gas phase. Fortunately, the altered identity of the compound in no way affects our high-level calculations on the annulene structure. Moreover, our conclusion based on the NMR data, namely, that the terminal methyl groups of the chains loop within a micelle so as to contact the micelle surface, remains valid, although the micelles are now more classical in nature than we had previously envisioned.1