Multiple CH Activations To Construct Biologically Active Molecules in a Process Completely Free of Organohalogen and Organometallic Components†
Bi-Jie Li
College of Chemistry, Peking University, Beijing 100871, China
Search for more papers by this authorShi-Liang Tian
College of Chemistry, Peking University, Beijing 100871, China
Search for more papers by this authorZhao Fang
College of Chemistry, Peking University, Beijing 100871, China
Search for more papers by this authorZhang-Jie Shi
Beijing National Laboratory of Molecular Sciences (BNLMS), PKU Green Chemistry Centre and Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry, Peking University, Beijing 100871, China, http://www.shigroup.cn/
State Key Laboratory of Organometallic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China, Fax: (+86) 10-6276-0890)
Search for more papers by this authorBi-Jie Li
College of Chemistry, Peking University, Beijing 100871, China
Search for more papers by this authorShi-Liang Tian
College of Chemistry, Peking University, Beijing 100871, China
Search for more papers by this authorZhao Fang
College of Chemistry, Peking University, Beijing 100871, China
Search for more papers by this authorZhang-Jie Shi
Beijing National Laboratory of Molecular Sciences (BNLMS), PKU Green Chemistry Centre and Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry, Peking University, Beijing 100871, China, http://www.shigroup.cn/
State Key Laboratory of Organometallic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China, Fax: (+86) 10-6276-0890)
Search for more papers by this authorSupport of this work by a starter grant from Peking University and the grant from National Sciences Foundation of China (No. 20542001, 20521202, 20672006) is gratefully acknowledged.
Graphical Abstract
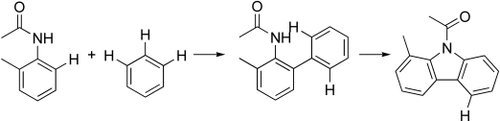
Step by step: Highly selective cross dehydrogenative arylation of acetanilides was developed to construct biaryls under mild condition. With this method, different aryl CH bonds were activated in sequential reactions to construct functionalized carbazoles (see scheme), which are present as key structural units in various biological molecules and organic optical materials.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2008/z704092_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aS. Murai, Activation of Unreactive Bonds and Organic Synthesis, Springer, Berlin, 1999, pp. 48–78;
- 1bA. E. Shilov, G. B. Shul'pin, Chem. Rev. 1997, 97, 2879;
- 1cF. Kakiuchi, N. Chatani, Adv. Synth. Catal. 2003, 345, 1077;
- 1dG. Dyker, Angew. Chem. 1999, 111, 1808;
10.1002/(SICI)1521-3757(19990614)111:12<1808::AID-ANGE1808>3.0.CO;2-V Google ScholarAngew. Chem. Int. Ed. 1999, 38, 1698;10.1002/(SICI)1521-3773(19990614)38:12<1698::AID-ANIE1698>3.0.CO;2-6 CAS PubMed Web of Science® Google Scholar
- 1eS. S. Stahl, J. A. Labinger, J. E. Bercaw, Angew. Chem. 1998, 110, 2298;
10.1002/(SICI)1521-3757(19980817)110:16<2298::AID-ANGE2298>3.0.CO;2-Y Google ScholarAngew. Chem. Int. Ed. 1998, 37, 2180;10.1002/(SICI)1521-3773(19980904)37:16<2180::AID-ANIE2180>3.0.CO;2-A CAS PubMed Web of Science® Google Scholar
- 1fA. R. Dick, M. S. Sanford, Tetrahedron 2006, 62, 2439;
- 1gB. A. Arndtsen, R. G. Bergman, T. A. Mobley, T. H. Peterson, Acc. Chem. Res. 1995, 28, 154;
- 1hJ. A. Labinger, J. E. Bercaw, Nature 2002, 417, 507;
- 1iY. Guari, S. Sabo-Etienne, B. Chaudret, Eur. J. Inorg. Chem. 1999, 1047.
10.1002/(SICI)1099-0682(199907)1999:7<1047::AID-EJIC1047>3.0.CO;2-B CAS Web of Science® Google Scholar
- 2
- 2aV. Ritleng, C. Sirlin, M. Pfeffer, Chem. Rev. 2002, 102, 1731;
- 2bJ. Dupont, C. S. Consorti, J. Spencer, Chem. Rev. 2005, 105, 2527;
- 2cS. Murai, F. Kakiuchi, S. Sekine, Y. Tanaka, A. Kamatani, M. Sonoda, N. Chatani, Nature 1993, 366, 529;
- 2dS. Oi, Y. Ogino, S. Fukita, Y. Inoue, Org. Lett. 2002, 4, 1783;
- 2eK. L. Tan, R. G. Bergman, J. A. Ellman, J. Am. Chem. Soc. 2002, 124, 13964;
- 2fC.-H. Jun, J.-B. Hong, Y.-H. Kim, K.-Y. Chung, Angew. Chem. 2000, 112, 3582;
10.1002/1521-3757(20001002)112:19<3582::AID-ANGE3582>3.0.CO;2-U Google ScholarAngew. Chem. Int. Ed. 2000, 39, 3440;10.1002/1521-3773(20001002)39:19<3440::AID-ANIE3440>3.0.CO;2-1 CAS PubMed Web of Science® Google Scholar
- 2gJ.-Y. Cho, M. K. Tse, D. Holmes, R. E. Maleczka, M. R. Smith III, Science 2002, 295, 305;
- 2hH. Y. Chen, S. Schlecht, T. C. Semple, J. F. Hartwig, Science 2000, 287, 1995;
- 2iC. Jia, D. Piao, J. Oyamada, W. Lu, T. Kitamura, Y. Fujiwara, Science 2000, 287, 1992.
- 3
- 3aM. D. K. Boele, G. P. F. van Strijdonck, A. H. M. de Vries, P. C. J. Kamer, J. G. de Vries, P. W. N. M. van Leeuwen, J. Am. Chem. Soc. 2002, 124, 1586;
- 3bV. G. Zaitsev, O. Daugulis, J. Am. Chem. Soc. 2005, 127, 4156;
- 3cM. T. Reetz, K. Sommer, Eur. J. Org. Chem. 2003, 3485;
- 3dJ. Zhao, M. Campo, R. C. Larock, Angew. Chem. 2005, 117, 1907; Angew. Chem. Int. Ed. 2005, 44, 1873;
- 3eX. Chen, J.-J. Li, X.-S. Hao, C. E. Goodhue, J.-Q. Yu, J. Am. Chem. Soc. 2006, 128, 78;
- 3fD. Kalyani, N. R. Deprez, L. V. Desai, M. S. Sanford, J. Am. Chem. Soc. 2005, 127, 7330;
- 3gB. S. Lane, M. A. Brown, D. Sames, J. Am. Chem. Soc. 2005, 127, 8050;
- 3hR. K. Thalji, J. A. Ellman, R. G. Bergman, J. Am. Chem. Soc. 2004, 126, 7192.
- 4C. Jia, T. Kitamura, Y. Fujiwara, Acc. Chem. Res. 2001, 34, 633.
- 5K. Godula, D. Sames, Science 2006, 312, 67.
- 6 Metal-Catalyzed Cross-Coupling Reactions (Eds.: ), Wiley-VCH, New York, 1998.
- 7
- 7aD. Alberico, M. E. Scott, M. Lautens, Chem. Rev. 2007, 107, 174;
- 7bT. Satoh, Y. Kawamura, M. Miura, M. Nomura, Angew. Chem. 1997, 109, 1820;
10.1002/ange.19971091623 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 1740;
- 7cL. Ackermann, A. Althammer, R. Born, Angew. Chem. 2006, 118, 2681; Angew. Chem. Int. Ed. 2006, 45, 2619;
- 7dJ. Zhao, D. Yue, M. A. Campo, R. C. Larock, J. Am. Chem. Soc. 2007, 129, 5288;
- 7eK.-I. Fujita, M. Nonogawa, R. Yamaguchi, Chem. Commun. 2004, 1926;
- 7fO. Daugulis, V. G. Zaitsev, D. Shabashov, Q. N. Pham, A. Lazareva, Synlett 2006, 3382;
- 7gR. Giri, N. Maugel, J.-J. Li, D.-H. Wang, S. P. Breazzano, L. B. Saunders, J.-Q. Yu, J. Am. Chem. Soc. 2007, 129, 3510;
- 7hF. Kakiuchi, S. Kan, K. Igi, N. Chatani, S. Murai, J. Am. Chem. Soc. 2003, 125, 1698;
- 7iS. Yang, B. Li, X. Wan, Z. Shi, J. Am. Chem. Soc. 2007, 129, 6066;
- 7jS. Oi, S. Fukita, Y. Inoue, Chem. Commun. 1998, 2439.
- 8
- 8aK. L. Hull, E. L. Lanni, M. S. Sanford, J. Am. Chem. Soc. 2006, 128, 14047;
- 8bK. Masui, H. Ikegami, A. Mori, J. Am. Chem. Soc. 2004, 126, 5074;
- 8cH. Iataaki, H. Yoshimoto, J. Org. Chem. 1973, 38, 76.
10.1021/jo00941a014 Google Scholar
- 9
- 9aZ. Li, C.-J. Li, J. Am. Chem. Soc. 2005, 127, 3672;
- 9bZ. Li, C.-J. Li, J. Am. Chem. Soc. 2005, 127, 6968.
- 10R. Li, L. Jiang, W. Lu, Organometallics 2006, 25, 5973.
- 11D. R. Stuart, K. Fagnou, Science 2007, 316, 1172.
- 12T. A. Dwight, N. R. Rue, D. Charyk, R. Josselyn, B. DeBoef, Org. Lett. 2007, 9, 3137.
- 13
- 13aX. Wan, Z. Ma, B. Li, K. Zhang, S. Cao, S. Zhang, Z. Shi, J. Am. Chem. Soc. 2006, 128, 7416;
- 13bZ. Shi, B. Li, X. Wan, J. Cheng, Z. Fang, B. Cao, C. Qin, Y. Wang, Angew. Chem. 2007, 119, 5650; Angew. Chem. Int. Ed. 2007, 46, 5554.
- 14
- 14aT. Ishiyama, K. Sato, Y. Nishio, N. Miyaura, Angew. Chem. 2003, 115, 5504;
10.1002/ange.200352399 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 5346;
- 14bN. Tsukada, T. Mitsuboshi, H. Setoguchi, Y. Inoue, J. Am. Chem. Soc. 2003, 125, 12102, and references therein.
- 15
- 15aD. García-Cuadrado, A. A. C. Braga, F. Maseras, A. M. Echavarren, J. Am. Chem. Soc. 2006, 128, 1066;
- 15bM. Lafrance, C. N. Rowley, T. K. Woo, K. Fagnou, J. Am. Chem. Soc. 2006, 128, 8754.
- 16H. J. Knölker, K. R. Reddy, Chem. Rev. 2002, 102, 4303.
- 17W. C. P. Tsang, N. Zheng, S. L. Buchwald, J. Am. Chem. Soc. 2005, 127, 14560.
- 18B. K. Chowdhury, S. Jha, Synth. Commun. 2001, 31, 1559.
Citing Literature
January 25, 2008
Pages 1115-1118