Three Groups Good, Four Groups Bad? Atropisomerism in ortho-Substituted Diaryl Ethers†
Mark S. Betson Dr.
School of Chemistry, University of Manchester, Oxford Road, Manchester M13 9PL, UK, Fax: (+44) 161-275-4939
Search for more papers by this authorJonathan Clayden Prof.
School of Chemistry, University of Manchester, Oxford Road, Manchester M13 9PL, UK, Fax: (+44) 161-275-4939
Search for more papers by this authorChristopher P. Worrall
School of Chemistry, University of Manchester, Oxford Road, Manchester M13 9PL, UK, Fax: (+44) 161-275-4939
Search for more papers by this authorSimon Peace Dr.
GlaxoSmithKline Medicines Research Centre, Gunnels Wood Road, Stevenage, Herts SG1 2NY, UK
Search for more papers by this authorMark S. Betson Dr.
School of Chemistry, University of Manchester, Oxford Road, Manchester M13 9PL, UK, Fax: (+44) 161-275-4939
Search for more papers by this authorJonathan Clayden Prof.
School of Chemistry, University of Manchester, Oxford Road, Manchester M13 9PL, UK, Fax: (+44) 161-275-4939
Search for more papers by this authorChristopher P. Worrall
School of Chemistry, University of Manchester, Oxford Road, Manchester M13 9PL, UK, Fax: (+44) 161-275-4939
Search for more papers by this authorSimon Peace Dr.
GlaxoSmithKline Medicines Research Centre, Gunnels Wood Road, Stevenage, Herts SG1 2NY, UK
Search for more papers by this authorWe are grateful to the Leverhulme Trust, GlaxoSmithKline, and the EPSRC for funding this study.
Graphical Abstract
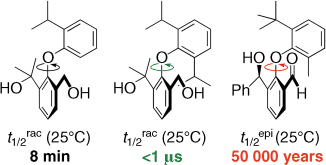
Bring on the substitute: Even outside of macrocyclic structures, such as vancomycin, appropriate substitution can give rise to atropisomerism in diaryl ethers. Stereochemical stability about the ArOAr axis at room temperature or above is possible when neither of the rings is symmetrically substituted and when at least one ring carries an ortho tert-butyl group or equivalent.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2006/z601866_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1K. C. Nicolaou, C. N. C. Boddy, S. Bräse, N. Winssinger, Angew. Chem. 1999, 111, 2230;
10.1002/(SICI)1521-3757(19990802)111:15<2230::AID-ANGE2230>3.0.CO;2-V Google ScholarAngew. Chem. Int. Ed. 1999, 38, 2096;10.1002/(SICI)1521-3773(19990802)38:15<2096::AID-ANIE2096>3.0.CO;2-F CAS PubMed Web of Science® Google ScholarB. M. Crowley, D. L. Boger, J. Am. Chem. Soc. 2006, 128, 2885, and references therein.
- 2E. L. Eliel, S. H. Wilen, Stereochemistry of Organic Compounds, Wiley, New York, 1994.
- 3S. Boidnard, L. Neuville, M. Bois-Choussy, J. Zhu, Org. Lett. 2000, 2, 2459.
- 4E. A. Couladouros, E. N. Pitsinos, V. I. Moutsos, G. Sarakinos, Chem. Eur. J. 2005, 11, 406.
- 5aJ. A. McRae, R. Y. Moir, J. J. Ursprung, H. H. Gibbs, J. Org. Chem. 1954, 19, 1500;
- 5bM. Dahlgard, R. Q. Brewster, J. Am. Chem. Soc. 1958, 80, 5861.
- 6H. Kessler, A. Rieker, W. Rundel, J. Chem. Soc. Chem. Commun. 1968, 475;
J. J. Bergman, W. D. Chandlwe, Can. J. Chem. 1972, 50, 353;
P. A. Lehman, Org. Magn. Reson. 1970, 2, 467.
10.1002/mrc.1270020505 Google Scholar
- 7B. M. Duggan, D. J. Craik, J. Med. Chem. 1997, 40, 2259.
- 8K. Fuji, T. Oka, T. Kawabata, T. Kinoshita, Tetrahedron Lett. 1998, 39, 1373.
- 9F. Theil, Angew. Chem. 1999, 111, 2493;
10.1002/(SICI)1521-3757(19990816)111:16<2493::AID-ANGE2493>3.0.CO;2-C Google ScholarAngew. Chem. Int. Ed. 1999, 38, 2345;10.1002/(SICI)1521-3773(19990816)38:16<2345::AID-ANIE2345>3.0.CO;2-5 CAS PubMed Web of Science® Google ScholarJ. S. Sawyer, Tetrahedron 2000, 56, 5045; Z. Liu, R. C. Larock, Org. Lett. 2004, 6, 99; D. A. Evans, J. L. Katz, T. R. West, Tetrahedron Lett. 1998, 39, 2937; D. M. T. Chan, K. L. Monaco, R. Wang, M. P. Winters, Tetrahedron Lett. 1998, 39, 2933; G. Mann, J. F. Hartwig, Tetrahedron Lett. 1997, 38, 8005.
- 10T. D. Krizan, J. C. Martin, J. Org. Chem. 1982, 47, 2681.
- 11For the use of (−)-ephedrine-derived oxazolidines as protecting groups for aldehydes during lithiation, see: J. Clayden, Y. J. Y. Foricher, M. Helliwell, P. Johnson, D. Mitjans, V. Vinader, Org. Biomol. Chem. 2006, 4, 444.
- 12J. Clayden in Chemistry of Organolithium Compounds, Vol. 1 (Eds.: ), Wiley, Chichester, 2004, p. 495;
10.1002/047002111X.ch10 Google ScholarH. W. Gschwend, H. R. Rodriguez, Org. React. 1979, 26, 1.
- 13M. S. Betson, J. Clayden, Synlett 2006, 745.
- 14Lineshapes at a range of temperatures close to, above, and below the Tc value were simulated by using gNMR software (Adept Scientific).
- 15The half-lives are for the approach to the equilibrium mixture, not half-lives for bond rotation. The bond rotation monitored in 6 c, 7 c, and 8 c does not in fact lead to interconversion of stereoisomers, but the half-life value is calculated in the same way for consistency.
- 16For examples in which trigonal substituents provide low barriers to bond rotation, see: A. I. Meyers, J. R. Flisak, R. A. Aitken, J. Am. Chem. Soc. 1987, 109, 5446; A. Ahmed, R. A. Bragg, J. Clayden, L. W. Lai, C. McCarthy, J. H. Pink, N. Westlund, S. A. Yasin, Tetrahedron 1998, 54, 13277; K. Kamikawa, M. Uemura, Synlett 2000, 938.
- 17Oki has suggested that atropisomers be defined as conformers that interconvert with a half-life of more than 1000 s−1: M. Oki, Top. Stereochem. 1983, 14, 1.
- 18D. L. Boger, J.-H. Weng, S. Miyazaki, J. J. McAtee, S. L. Castle, S. H. Kim, Y. Mori, O. Rogel, H. Strittmatter, Q. Jin, J. Am. Chem. Soc. 2000, 122, 10047.
- 19R. Adams, H. C. Yuan, Chem. Rev. 1933, 33, 261.
10.1021/cr60042a003 Google Scholar
- 20This estimation is made on the assumption that at least one pair of diastereotopic or more or less equally populated diastereoisomeric peaks would have a peak separation of >0.1 ppm at the slow exchange limit.
- 21Substituents attached to the symmetrical ring may show coalescences at higher temperatures, which is indicative of much higher barriers, but these must be barriers to nonconcerted bond rotations; a full discussion will follow in a later report.
- 22F. Mohmadi, N. G. J. Richards, W. C. Guida, R. Liskamp, M. Lipton, C. Caufield, G. Chang, T. Hendrickson, W. C. Still, J. Comput. Chem. 1990, 11, 440.
- 23P. A. Lehman, E. C. Jorgensen, Tetrahedron 1965, 21, 363; G. Montaudo, P. Finocchiaro, E. Trivellone, F. Bottino, P. Maravigna, Tetrahedron 1971, 27, 2125; J. C. Emmett, E. S. Pepper, Nature 1975, 257, 334.
- 24Ground-state destabilization of tetra-ortho-substituted relative to tri-ortho-substituted diaryl ethers may also play a role in lowering the barrier, but the results presented in Table 1, entries 22–25 suggest that it is not an important one.
- 25For a discussion of geared rotation in related compounds, see: J. Clayden, J. H. Pink, Angew. Chem. 1998, 110, 2040;
10.1002/(SICI)1521-3757(19980703)110:13/14<2040::AID-ANGE2040>3.0.CO;2-Y Web of Science® Google ScholarAngew. Chem. Int. Ed. 1998, 37, 1937;10.1002/(SICI)1521-3773(19980803)37:13/14<1937::AID-ANIE1937>3.0.CO;2-4 CAS Web of Science® Google ScholarR. A. Bragg, J. Clayden, Org. Lett. 2000, 2, 3351; R. A. Bragg, J. Clayden, G. A. Morris, J. H. Pink, Chem. Eur. J. 2002, 8, 1279; for a more general discussion of gearing effects, see:10.1002/1521-3765(20020315)8:6<1279::AID-CHEM1279>3.0.CO;2-7 CAS PubMed Web of Science® Google ScholarG. S. Kottas, L. I. Clarke, D. Horinek, J. Michl, Chem. Rev. 2005, 105, 1281; H. Iwamura, K. Mislow, Acc. Chem. Res. 1988, 21, 175.