Total Synthesis of Iejimalide B†
Alois Fürstner Prof.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorCristina Nevado Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorMartin Tremblay Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorCarine Chevrier Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorFilip Teplý Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorChristophe Aïssa Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorMario Waser Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorAlois Fürstner Prof.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorCristina Nevado Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorMartin Tremblay Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorCarine Chevrier Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorFilip Teplý Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorChristophe Aïssa Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorMario Waser Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorGenerous financial support from the MPG, the Fonds der Chemischen Industrie, the Alexander-von-Humboldt Foundation (fellowship to C.N.), the NSERC Canada (fellowship to M.T.), and the Austrian Fonds zur Förderung der Wissenschaftlichen Forschung (fellowship to M.W.) is gratefully acknowledged. We thank Dr. R. Mynott, B. Gabor, and C. Wirtz for their invaluable help with the structure assignment of several key compounds, and R. Leichtweiß for HPLC assistance.
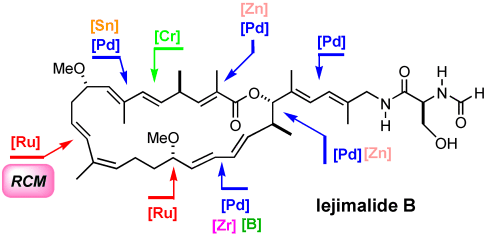
Graphical Abstract
Metathesis to the rescue: Although counterintuitive at first sight, application of ring-closing metathesis (RCM) to a cyclization precursor containing 10 double bonds has led to the selective and high-yielding formation of the macrocyclic core of iejimalide B, a potent cytotoxic agent of marine origin. In contrast, a macrolactonization approach failed to afford this intricate target.
References
- 1
- 1aK. Nozawa, M. Tsuda, H. Ishiyama, T. Sasaki, T. Tsuruo, J. Kobayashi, Bioorg. Med. Chem. 2006, 14, 1063–1067;
- 1bthe cytotoxicity data are available from the NCI homepage (http://www.dtp.nci.nih.gov/docs/dtp search.html).
- 2
- 2aJ. Kobayashi, J. Cheng, T. Ohta, H. Nakamura, S. Nozoe, Y. Hirata, Y. Ohizumi, T. Sasaki, J. Org. Chem. 1988, 53, 6147–6150;
- 2bY. Kikuchi, M. Ishibashi, T. Sasaki, J. Kobayashi, Tetrahedron Lett. 1991, 32, 797–798;
- 2cM. Tsuda, K. Nozawa, K. Shimbo, H. Ishiyama, E. Fukushi, J. Kawabata, J. Kobayashi, Tetrahedron Lett. 2003, 44, 1395–1399.
- 3For previous synthetic efforts see:
- 3aM. Cottard, N. Kann, T. Rein, B. Åkermark, P. Helquist, Tetrahedron Lett. 1995, 36, 3115–3118;
- 3bM. T. Mendlik, M. Cottard, T. Rein, P. Helquist, Tetrahedron Lett. 1997, 38, 6375–6378;
- 3cT. M. Pedersen, E. L. Hansen, J. Kane, T. Rein, P. Helquist, P.-O. Norrby, D. Tanner, J. Am. Chem. Soc. 2001, 123, 9738–9742.
- 4A. Fürstner, C. Aïssa, C. Chevrier, F. Teplý, C. Nevado, M. Tremblay, Angew. Chem. 2006, 118, 5964–5969; Angew. Chem. Int. Ed. 2006, 45, 5832–5837.
- 5
- 5aT. M. Trnka, R. H. Grubbs, Acc. Chem. Res. 2001, 34, 18–29;
- 5bA. Fürstner, Angew. Chem. 2000, 112, 3140–3172;
10.1002/1521-3757(20000901)112:17<3140::AID-ANGE3140>3.0.CO;2-G Google ScholarAngew. Chem. Int. Ed. 2000, 39, 3012–3043;10.1002/1521-3773(20000901)39:17<3012::AID-ANIE3012>3.0.CO;2-G CAS PubMed Web of Science® Google Scholar
- 5cR. R. Schrock, A. H. Hoveyda, Angew. Chem. 2003, 115, 4740–4782;
10.1002/ange.200300576 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 4592–4633;
- 5dA. Deiters, S. F. Martin, Chem. Rev. 2004, 104, 2199–2238;
- 5eK. C. Nicolaou, P. G. Bulger, D. Sarlah, Angew. Chem. 2005, 117, 4564–4601;
10.1002/ange.200500369 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 4490–4527;
- 5fM. Schuster, B. Blechert, Angew. Chem. 1997, 109, 2124–2144; Angew. Chem. Int. Ed. Engl. 1997, 36, 2036–2056.
- 6Selected examples:
- 6aC. Aïssa, R. Riveiros, J. Ragot, A. Fürstner, J. Am. Chem. Soc. 2003, 125, 15512–15520;
- 6bA. Fürstner, F. Jeanjean, P. Razon, C. Wirtz, R. Mynott, Chem. Eur. J. 2003, 9, 320–326;
- 6cA. Fürstner, K. Radkowski, C. Wirtz, R. Goddard, C. W. Lehmann, R. Mynott, J. Am. Chem. Soc. 2002, 124, 7061–7069;
- 6dA. Fürstner, T. Dierkes, O. R. Thiel, G. Blanda, Chem. Eur. J. 2001, 7, 5286–5298;
10.1002/1521-3765(20011217)7:24<5286::AID-CHEM5286>3.0.CO;2-G CAS PubMed Web of Science® Google Scholar
- 6eA. Fürstner, T. Müller, J. Am. Chem. Soc. 1999, 121, 7814–7821;
- 6fB. Scheiper, F. Glorius, A. Leitner, A. Fürstner, Proc. Natl. Acad. Sci. USA 2004, 101, 11960–11965;
- 6gA. Fürstner, C. Müller, Chem. Commun. 2005, 5583–5585.
- 7For a pertinent example of an attempted RCM prohibited by intervention of a 1,3-diene unit see:
- 7aA. K. Ghosh, G. Gong, J. Org. Chem. 2006, 71, 1085–1093. For recent total syntheses involving metathesis in the presence of 1,3-dienes that remain untouched see:
- 7bT. Yoshimura, F. Yakushiji, S. Kondo, X. Wu, M. Shindo, K. Shishido, Org. Lett. 2006, 8, 475–478;
- 7cB. Wu, Q. Liu, G. A. Sulikowski, Angew. Chem. 2004, 116, 6841–6843; Angew. Chem. Int. Ed. 2004, 43, 6673–6675;
- 7dD. J. Kerr, A. C. Willis, B. L. Flynn, Org. Lett. 2004, 6, 457–460;
- 7eR. E. Maleczka, Jr.,L. R. Terrell, F. Geng, J. S. Ward, Org. Lett. 2002, 4, 2841–2844.
- 8
- 8aFor a particularly instructive case see: K. Biswas, H. Lin, J. T. Njardarson, M. D. Chappell, T.-C. Chou, Y. Guan, W. P. Tong, L. He, S. B. Horwitz, S. J. Danishefsky, J. Am. Chem. Soc. 2002, 124, 9825–9832;
- 8ba discussion and a comprehensive literature coverage of metathetic diene–ene cyclizations is found in: F. Lacombe, K. Radkowski, G. Seidel, A. Fürstner, Tetrahedron 2004, 60, 7315–7324.
- 9
- 9aA. Fürstner, K. Langemann, J. Org. Chem. 1996, 61, 3942–3943;
- 9bA. Fürstner, K. Langemann, Synthesis 1997, 792–803;
- 9cA. Fürstner, K. Langemann, J. Am. Chem. Soc. 1997, 119, 9130–9136.
- 10A. Suzuki, J. Organomet. Chem. 1999, 576, 147–168.
- 11
- 11aA. Arase, H. Hoshi, A. Mijin, K. Nishi, Synth. Commun. 1995, 25, 1957–1962;
- 11bK. C. Nicolaou, K. C. Fylaktakidou, H. Monenschein, Y. Li, B. Weyershausen, H. J. Mitchell, H. Wei, P. Guntupalli, D. Hepworth, K. Sugita, J. Am. Chem. Soc. 2003, 125, 15433–15442.
- 12
- 12aJ. A. Marshall, Chem. Rev. 2000, 100, 3163–3185;
- 12bJ. A. Marshall, N. D. Adams, J. Org. Chem. 1999, 64, 5201–5204;
- 12crecent application: O. Lepage, E. Kattnig, A. Fürstner, J. Am. Chem. Soc. 2004, 126, 15970–15971.
- 13D. W. Hart, J. Schwartz, J. Am. Chem. Soc. 1974, 96, 8115–8116.
- 14A. Gopalarathnam, S. G. Nelson, Org. Lett. 2006, 8, 7–10.
- 15Small amounts (10–15 %) of the regioisomeric alkenylstannane could be separated by flash chromatography. The corresponding pinacolborane was also prepared but failed to undergo productive cross-coupling with iodide 30.
- 16
- 16aS. M. Ceccarelli, U. Piarulli, J. Tesler, C. Gennari, Tetrahedron Lett. 2001, 42, 7421–7425;
- 16bD. Horne, J. Gaudino, W. J. Thompson, Tetrahedron Lett. 1984, 25, 3529–3532.
- 17D. L. Comins, A. Dehghani, Tetrahedron Lett. 1992, 33, 6299–6302.
- 18E. Negishi, X. Zheng, Z. Tan, M. Qian, Q. Hu, Z. Huang in Metal-Catalyzed Cross-Coupling Reactions, Vol. 2, 2nd ed. ), Wiley-VCH, Weinheim, 2004, pp. 815–889.
10.1002/9783527619535.ch15 Google Scholar
- 19This route to 27 was chosen for two reasons: first, problems with partial racemization were encountered when the aldehyde derived from 23 was treated with Wittig reagents in an attempt to form 28 directly; secondly, it is known that iejimalide B bearing a methyl group at C2 is more active than iejimalide A which features a proton at this position, see Ref. [2]; the chosen route via triflate 27 allows for systematic variations at this position.
- 20
- 20aK. Takai, K. Nitta, K. Utimoto, J. Am. Chem. Soc. 1986, 108, 7408–7410;
- 20bD. A. Evans, W. C. Black, J. Am. Chem. Soc. 1993, 115, 4497–4513;
- 20creview: A. Fürstner, Chem. Rev. 1999, 99, 991–1045.
- 21Worried that reversible double-bond isomerizations at the aldehyde stage or an equilibrium between 30 and 31 would racemize the chiral center at C4, the ee value of compound 30 was carefully checked and found to be ≥90 %.
- 22P. Espinet, A. M. Echavarren, Angew. Chem. 2004, 116, 4808–4839;
10.1002/ange.200300638 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 4704–4734.
- 23
- 23aH. Fuwa, N. Kainuma, K. Tachibana, M. Sasaki, J. Am. Chem. Soc. 2002, 124, 14983–14992;
- 23bfor the use of CuTC in Pd-free Stille reactions see: G. D. Allred, L. S. Liebeskind, J. Am. Chem. Soc. 1996, 118, 2748–2749;
- 23cT. B. Durham, N. Blanchard, B. M. Savall, N. A. Powell, W. R. Roush, J. Am. Chem. Soc. 2004, 126, 9307–9317.
- 24M. Hikota, Y. Sakurai, K. Horita, O. Yonemitsu, Tetrahedron Lett. 1990, 31, 6367–6370.
- 25
- 25aM. Scholl, S. Ding, C. W. Lee, R. H. Grubbs, Org. Lett. 1999, 1, 953–956;
- 25bsee also: J. Huang, E. D. Stevens, S. P. Nolan, J. L. Pedersen, J. Am. Chem. Soc. 1999, 121, 2674–2678;
- 25cL. Ackermann, A. Fürstner, T. Weskamp, F. J. Kohl, W. A. Herrmann, Tetrahedron Lett. 1999, 40, 4787–4790.
- 26
- 26aM. Sakaitani, Y. Ohfune, J. Org. Chem. 1990, 55, 870–876;
- 26bM. Nazaré, H. Waldmann, Chem. Eur. J. 2001, 7, 3363–3376;
10.1002/1521-3765(20010803)7:15<3363::AID-CHEM3363>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar
- 26cB. M. Trost, D. R. Fandrick, Org. Lett. 2005, 7, 823–826.
- 27D. R. Hill, C.-N. Hsiao, R. Kurukulasuriya, S. J. Wittenberger, Org. Lett. 2002, 4, 111–113.
- 28Uncontrolled isomerizations were observed even at 35 °C.
- 29Significant amounts of the symmetrical anhydride derived from acid 33 could even be isolated from the mixture; for precedents and a mechanistic discussion see: I. Dhimitruka, J. SantaLucia, Jr., Org. Lett. 2006, 8, 47–50.