Studies on Iejimalide B: Preparation of the Seco Acid and Identification of the Molecule's “Achilles Heel”†
Alois Fürstner Prof.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorChristophe Aïssa Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorCarine Chevrier Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorFilip Teplý Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorCristina Nevado Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorMartin Tremblay Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorAlois Fürstner Prof.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorChristophe Aïssa Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorCarine Chevrier Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorFilip Teplý Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorCristina Nevado Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorMartin Tremblay Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorGenerous financial support from the MPG, the Fonds der Chemischen Industrie, the Alexander-von-Humboldt Foundation (fellowship to C.N.), and the NSERC, Canada (fellowship to M.T.) is gratefully acknowledged. We thank Dr. R. Riveiros for assistance in the initial phase of this project, and Dr. R. Mynott, B. Gabor, and C. Wirtz for their invaluable help with the structure assignment of several key compounds.
Graphical Abstract
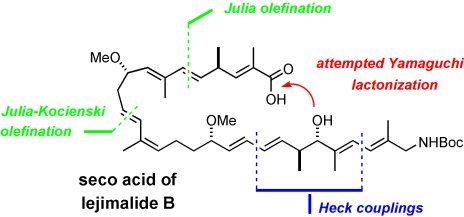
Limitations of models in total synthesis are illustrated by a study towards the potent cytotoxic macrolide iejimalide B. Although the Yamaguchi protocol allowed for the esterification of elaborate segments, attempted macrolactonization of the seco acid met with failure (see scheme, Boc= tert-butyloxycarbonyl). The assembly of the seco acid involves some of the most advanced applications of the Julia olefination known to date.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2006/z601859_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. Kobayashi, J. Cheng, T. Ohta, H. Nakamura, S. Nozoe, Y. Hirata, Y. Ohizumi, T. Sasaki, J. Org. Chem. 1988, 53, 6147–6150;
- 1bY. Kikuchi, M. Ishibashi, T. Sasaki, J. Kobayashi, Tetrahedron Lett. 1991, 32, 797–798.
- 2
- 2aK. Nozawa, M. Tsuda, H. Ishiyama, T. Sasaki, T. Tsuruo, J. Kobayashi, Bioorg. Med. Chem. 2006, 14, 1063–1067;
- 2bM. Tsuda, K. Nozawa, K. Shimbo, H. Ishiyama, E. Fukushi, J. Kawabata, J. Kobayashi, Tetrahedron Lett. 2003, 44, 1395–1399.
- 3The activity data are available from the NCI homepage (http://www.dtp.nci.nih.gov/docs/dtp_search.html).
- 4Although the NCI data show no particular selectivity, a preliminary report from the Walther Cancer Research Center (http://www.nd.edu/≈science/documents/waltherbrochure.pdf) claims spectacular effects against colon cancer and stunning morphological changes upon injection of iejimalides into solid tumors.
- 5For a related endeavor see: A. Fürstner, D. Kirk, M. D. B. Fenster, C. Aïssa, D. De Souza, O. Müller, Proc. Natl. Acad. Sci. USA 2005, 102, 8103–8108.
- 6A. Fürstner, C. Nevado, M. Tremblay, C. Chevrier, F. Teplý, C. Aïssa, M. Waser, Angew. Chem. 2006, 118, 5969–5974; Angew. Chem. Int. Ed. 2006, 45, 5837–5842.
- 7For fragment syntheses see:
- 7aM. Cottard, N. Kann, T. Rein, B. Åkermark, P. Helquist, Tetrahedron Lett. 1995, 36, 3115–3118;
- 7bM. T. Mendlik, M. Cottard, T. Rein, P. Helquist, Tetrahedron Lett. 1997, 38, 6375–6378;
- 7cT. M. Pedersen, E. L. Hansen, J. Kane, T. Rein, P. Helquist, P.-O. Norrby, D. Tanner, J. Am. Chem. Soc. 2001, 123, 9738–9742.
- 8
- 8aJ. Inanaga, K. Hirata, H. Saeki, T. Katsuki, M. Yamaguchi, Bull. Chem. Soc. Jpn. 1979, 52, 1989–1993;
- 8breview: A. Parenty, X. Moreau, J.-M. Campagne, Chem. Rev. 2006, 106, 911–939.
- 9For advanced applications from our research group see:
- 9aO. Lepage, E. Kattnig, A. Fürstner, J. Am. Chem. Soc. 2004, 126, 15970–15971;
- 9bJ. Mlynarski, J. Ruiz-Caro, A. Fürstner, Chem. Eur. J. 2004, 10, 2214–2222.
- 10Review: P. R. Blakemore, J. Chem. Soc. Perkin Trans. 1 2002, 2563–2585.
- 11
- 11aA. Fürstner, M. Albert, J. Mlynarski, M. Matheu, E. DeClercq, J. Am. Chem. Soc. 2003, 125, 13132–13142;
- 11bA. Fürstner, J. Mlynarski, M. Albert, J. Am. Chem. Soc. 2002, 124, 10274–10275;
- 11cA. Fürstner, F. Feyen, H. Prinz, H. Waldmann, Tetrahedron 2004, 60, 9543–9558.
- 12K. C. Nicolaou, P. G. Bulger, D. Sarlah, Angew. Chem. 2005, 117, 4516–4563;
10.1002/ange.200500368 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 4442–4489.
- 13For details see the Supporting Information.
- 14S. Bräse, A. de Meijere in Metal-Catalyzed Cross-Coupling Reactions, Vol. 1 (Eds.: ), Wiley-VCH, Weinheim, 2004, pp. 217–315.
10.1002/9783527619535.ch5 Google Scholar
- 15H. C. Brown, K. S. Bhat, J. Am. Chem. Soc. 1986, 108, 293–294.
- 16
- 16aK. Matsumura, S. Hashiguchi, T. Ikariya, R. Noyori, J. Am. Chem. Soc. 1997, 119, 8738–8739;
- 16bR. Noyori, S. Hashiguchi, Acc. Chem. Res. 1997, 30, 97–102;
- 16cfor a recent application from this research group see: A. Fürstner, P. Hannen, Chem. Eur. J. 2006, 12, 3006–3019.
- 17R. Suzuki, H. Tsukuda, N. Watanabe, Y. Kuwatani, I. Ueda, Tetrahedron 1998, 54, 2477–2496.
- 18W. C. Still, C. Gennari, Tetrahedron Lett. 1983, 24, 4405–4408.
- 19S. J. Mickel, G. H. Sedelmeier, D. Niederer, F. Schuerch, M. Seger, K. Schreiner, R. Daeffler, A. Osmani, D. Bixel, O. Loiseleur, J. Cercus, H. Stettler, K. Schaer, R. Gamboni, A. Bach, G.-P. Chen, W. Chen, P. Geng, G. T. Lee, E. Loeser, J. McKenna, F. R. Kinder, Jr.,K. Konigsberger, K. Prasad, T. M. Ramsey, N. Reel, O. Repiè, L. Rogers, W.-C. Shieh, R.-M. Wang, L. Waykole, S. Xue, G. Florence, I. Paterson, Org. Process Res. Dev. 2004, 8, 113–121.
- 20The use of TBAF and other fluoride sources scrambled the trisubstituted olefin of the enoate.
- 21D. W. Hart, J. Schwartz, J. Am. Chem. Soc. 1974, 96, 8115–8116.
- 22M. Kalesse, K. P. Chary, M. Quitschalle, A. Burzlaff, C. Kasper, T. Scheper, Chem. Eur. J. 2003, 9, 1129–1136.
- 23P. Gamez, I. W. C. E. Arends, R. A. Sheldon, J. Reedijk, Adv. Synth. Catal. 2004, 346, 805–811.
- 24P. R. Blakemore, W. J. Cole, P. J. Kocienski, A. Morley, Synlett 1998, 26–28.
- 25Note that an alcohol might interfere with the Smiles rearrangement, which constitutes the crucial second step of the Kocienski variant of the Julia olefination, see. Ref. [24].
- 26K. C. Nicolaou, A. A. Estrada, M. Zak, S. H. Lee, B. S. Safina, Angew. Chem. 2005, 117, 1402–1406;
10.1002/ange.200462207 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 1378–1382.
- 27Attempts to form the macrocycle by intramolecular transesterification following a closely related method (cat. Bu2SnO, toluene, reflux) were unsuccessful; compare: P. Baumhof, R. Mazitschek, A. Giannis, Angew. Chem. 2001, 113, 3784–3786;
10.1002/1521-3757(20011001)113:19<3784::AID-ANGE3784>3.0.CO;2-3 Google ScholarAngew. Chem. Int. Ed. 2001, 40, 3672–3674.10.1002/1521-3773(20011001)40:19<3672::AID-ANIE3672>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar
- 28M. Hikota, Y. Sakurai, K. Horita, O. Yonemitsu, Tetrahedron Lett. 1990, 31, 6367–6370.
- 29Attempts to suppress this pathway by replacement of DMAP with 2,6-di-tert-butylpyridine as a more bulky base were unsuccessful.
- 30Formation of phenols by electrocyclizations of polyunsaturated ketenes:
- 30aM. J. Heileman, H. W. Moore, Tetrahedron Lett. 1998, 39, 3643–3646;
- 30bW. F. Austin, Y. Zhang, R. L. Danheiser, Org. Lett. 2005, 7, 3905–3908.
- 31
- 31aT. M. Trnka, R. H. Grubbs, Acc. Chem. Res. 2001, 34, 18–29;
- 31bA. Fürstner, Angew. Chem. 2000, 112, 3140–3172;
10.1002/1521-3757(20000901)112:17<3140::AID-ANGE3140>3.0.CO;2-G Google ScholarAngew. Chem. Int. Ed. 2000, 39, 3012–3043.10.1002/1521-3773(20000901)39:17<3012::AID-ANIE3012>3.0.CO;2-G CAS PubMed Web of Science® Google Scholar