Radical Copolymerization of a Phosphaalkene with Styrene: New Phosphine-Containing Macromolecules and Their Use in Polymer-Supported Catalysis†
Chi-Wing Tsang Dr.
Department of Chemistry, University of British Columbia, 2036 Main Mall, Vancouver, British Columbia, V6T 1Z1, Canada, Fax: (+1) 604-822-2847
Search for more papers by this authorBaharnaz Baharloo
Department of Chemistry, University of British Columbia, 2036 Main Mall, Vancouver, British Columbia, V6T 1Z1, Canada, Fax: (+1) 604-822-2847
Search for more papers by this authorDavid Riendl
Department of Chemistry, University of British Columbia, 2036 Main Mall, Vancouver, British Columbia, V6T 1Z1, Canada, Fax: (+1) 604-822-2847
Search for more papers by this authorMandy Yam
Department of Chemistry, University of British Columbia, 2036 Main Mall, Vancouver, British Columbia, V6T 1Z1, Canada, Fax: (+1) 604-822-2847
Search for more papers by this authorDerek P. Gates Prof. Dr.
Department of Chemistry, University of British Columbia, 2036 Main Mall, Vancouver, British Columbia, V6T 1Z1, Canada, Fax: (+1) 604-822-2847
Search for more papers by this authorChi-Wing Tsang Dr.
Department of Chemistry, University of British Columbia, 2036 Main Mall, Vancouver, British Columbia, V6T 1Z1, Canada, Fax: (+1) 604-822-2847
Search for more papers by this authorBaharnaz Baharloo
Department of Chemistry, University of British Columbia, 2036 Main Mall, Vancouver, British Columbia, V6T 1Z1, Canada, Fax: (+1) 604-822-2847
Search for more papers by this authorDavid Riendl
Department of Chemistry, University of British Columbia, 2036 Main Mall, Vancouver, British Columbia, V6T 1Z1, Canada, Fax: (+1) 604-822-2847
Search for more papers by this authorMandy Yam
Department of Chemistry, University of British Columbia, 2036 Main Mall, Vancouver, British Columbia, V6T 1Z1, Canada, Fax: (+1) 604-822-2847
Search for more papers by this authorDerek P. Gates Prof. Dr.
Department of Chemistry, University of British Columbia, 2036 Main Mall, Vancouver, British Columbia, V6T 1Z1, Canada, Fax: (+1) 604-822-2847
Search for more papers by this authorThe Natural Sciences and Engineering Research Council (NSERC) of Canada, the Canada Foundation for Innovation (CFI)/New Opportunities, and the BC Ministry for Advanced Education and Technology are gratefully acknowledged for funding. We also thank Prof. Jennifer Love for useful discussions, Prof. John Scheffer for the use of his gas chromatograph, and Dr. Scott Clendenning (U. Toronto) for conducting the triple-detection GPC experiment.
Graphical Abstract
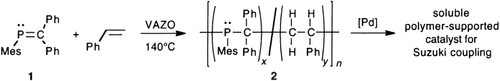
PC bonds mimic CC bonds in the radical-initiated copolymerization of a phosphaalkene 1 and styrene (see scheme). The resulting copolymers 2 have unprecedented phosphine-containing backbones with phosphorus compositions that vary depending on the monomer ratios. These novel functional hybrid inorganic–organic macromolecules are used as polymeric supports for the Pd-catalyzed Suzuki coupling. VAZO=1,1′-azobis(cyclohexanecarbonitrile).
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2004/z460939_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1G. Becker, Z. Anorg. Allg. Chem. 1976, 423, 242.
- 2K. B. Dillon, F. Mathey, J. F. Nixon, Phosphorus: The Carbon Copy, Wiley, New York, 1998.
- 3For general reviews of phosphaalkenes, see:
- 3aF. Mathey, Angew. Chem. 2003, 115, 1616;
10.1002/ange.200200557 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 1578;
- 3bL. Weber, Eur. J. Inorg. Chem. 2000, 2425;
- 3cM. Yoshifuji, J. Chem. Soc. Dalton Trans. 1998, 3343;
- 3dF. Mathey, Acc. Chem. Res. 1992, 25, 90;
- 3eR. Appel in Multiple Bonds and Low Coordination in Phosphorus Chemistry (Eds.: ), Thieme, Stuttgart, 1990, p. 157;
- 3fR. Appel, F. Knoll, Adv. Inorg. Chem. 1989, 33, 259;
- 3gJ. F. Nixon, Chem. Rev. 1988, 88, 1327;
- 3hR. Appel, F. Knoll, I. Ruppert, Angew. Chem. 1981, 93, 771; Angew. Chem. Int. Ed. Engl. 1981, 20, 731.
- 4For a review highlighting some potential applications of compounds containing PC bonds in catalysis, see: L. Weber, Angew. Chem. 2002, 114, 583; Angew . Chem. Int. Ed. 2002, 41, 563, and references therein.
- 5
- 5aT. C. Klebach, R. Lourens, F. Bickelhaupt, J. Am. Chem. Soc. 1978, 100, 4886;
- 5bT. A. van der Knaap, T. C. Klebach, F. Visser, F. Bickelhaupt, P. Ros, E. J. Baerends, C. H. Stam, M. Konijn, Tetrahedron 1984, 40, 765;
- 5cO. Mundt, G. Becker, W. Uhl, Z. Anorg. Allg. Chem. 1986, 540/ 541, 319.
10.1002/zaac.19865400935 Google Scholar
- 6C.-W. Tsang, M. Yam, D. P. Gates, J. Am. Chem. Soc. 2003, 125, 1480.
- 7For reviews, see:
- 7aJ. E. Mark, H. R. Allcock, R. West, Inorganic Polymers, Prentice-Hall, Englewood Cliffs, 1992;
- 7bH. R. Allcock, Chemistry and Applications of Polyphosphazenes, Wiley, New Jersey, 2003;
- 7cI. Manners, Angew. Chem. 1996, 108, 1712;
10.1002/ange.19961081504 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 1602;
- 7dR. H. Neilson, P. Wisian-Neilson, Chem. Rev. 1988, 88, 541;
- 7eA. R. McWilliams, H. Dorn, I. Manners, Top. Curr. Chem. 2002, 220, 141;
- 7fJ.-P. Majoral, A. M. Caminade, V. Maraval, Chem. Commun. 2002, 2929.
- 8For recent work on main-chain-phosphorus polymers, see:
- 8aR. C. Smith, J. D. Protasiewicz, J. Am. Chem. Soc. 2004, 126, 2268;
- 8bH. Dorn, J. M. Rodenzo, B. Brunnhöfer, E. Rivard, J. A. Massey, I. Manners, Macromolecules 2003, 36, 291;
- 8cY. Morisaki, Y. Aiki, Y. Chujo, Macromolecules 2003, 36, 2594;
- 8dV. A. Wright, D. P. Gates, Angew. Chem. 2002, 114, 2495;
Angew. Chem. Int. Ed. 2002, 41, 2389;
10.1002/1521-3773(20020703)41:13<2389::AID-ANIE2389>3.0.CO;2-6 CAS PubMed Web of Science® Google Scholar
- 8eH. R. Allcock, S. D. Reeves, C. R. de Denus, C. A. Crane, Macromolecules 2001, 34, 748;
- 8fC. H. Walker, J. V. St. John, P. Wisian-Neilson, J. Am. Chem. Soc. 2001, 123, 3846;
- 8gC. Hay, C. Fischmeister, M. Hissler, L. Toupet, R. Réau, Angew. Chem. 2000, 112, 1882;
10.1002/(SICI)1521-3757(20000515)112:10<1882::AID-ANGE1882>3.0.CO;2-1 Google ScholarAngew. Chem. Int. Ed. 2000, 39, 1812;10.1002/(SICI)1521-3773(20000515)39:10<1812::AID-ANIE1812>3.0.CO;2-D CAS PubMed Web of Science® Google Scholar
- 8hB. L. Lucht, N. O. St. Onge, Chem. Commun. 2000, 2097;
- 8iT. J. Peckham, J. A. Massey, C. H. Honeyman, I. Manners, Macromolecules 1999, 32, 2830;
- 8jS. S. H. Mao, T. D. Tilley, Macromolecules 1997, 30, 5566.
- 9The ratio of (aryl H):(alkyl H) for the two monomer units (i.e. 1 and styrene) in the 1H NMR spectrum is not very sensitive to large changes in the % incorporation of 1 into the copolymer 3. Therefore, we have found that 1H NMR spectroscopy does not give an accurate measure of the phosphaalkene content. For example, a polymer consisting entirely of the methylenephosphine unit would show a (aryl H):(alkyl H) ratio of 1.33 (i.e. 12 H/9 H), whereas the ratio for polystyrene would be 1.66 (5 H/3 H).
- 10See, for example: C. U. Pittman,Jr. in Comprehensive Organometallic Chemistry, Vol. 8 (Eds.: ), Pergamon, Oxford, 1982, p. 553;
10.1016/B978-008046518-0.00114-8 Google ScholarD. E. Bergbreiter, Chem. Rev. 2002, 102, 3345; T. J. Dickerson, N. N. Reed, K. D. Janda, Chem. Rev. 2002, 102, 3325; C. A. McNamara, M. J. Dixon, M. Bradley, Chem. Rev. 2002, 102, 3275; N. E. Leadbeater, M. Marco, Chem. Rev. 2002, 102, 3217; P. H. Toy, K. D. Janda, Acc. Chem. Res. 2000, 33, 546; S. J. Shuttleworth, S. M. Allin, P. K. Sharma, Synthesis 1997, 1217; S. J. Shuttleworth, S. M. Allin, R. D. Wilson, D. Nasturica, Synthesis 2000, 1035.
- 11For leading references on Suzuki coupling in the presence of molecular phosphines, see, for example:
- 11aN. Miyaura, A. Suzuki, Chem. Rev. 1995, 95, 2457;
- 11bA. F. Littke, G. C. Fu, Angew. Chem. 2002, 114, 4350;
10.1002/1521-3757(20021115)114:22<4350::AID-ANGE4350>3.0.CO;2-0 Google ScholarAngew. Chem. Int. Ed. 2002, 41, 4176;10.1002/1521-3773(20021115)41:22<4176::AID-ANIE4176>3.0.CO;2-U CAS PubMed Web of Science® Google Scholar
- 11cM. Miura, Angew. Chem. 2004, 116, 2251; Angew. Chem. Int. Ed. 2004, 43, 2201;
- 11dS. D. Walker, T. E. Barder, J. R. Martinelli, S. L. Buchwald, Angew. Chem. 2004, 116, 1907; Angew. Chem. Int. Ed. 2004, 43, 1871;
- 11eA. F. Littke, C. Dai, G. C. Fu, J. Am. Chem. Soc. 2000, 122, 4020.
- 12For Suzuki coupling in the presence of insoluble phosphine polymers/resins, see:
- 12aY. M. A. Yamada, K. Takeda, H. Takahashi, S. Ikegami, Org. Lett. 2002, 4, 3371;
- 12bT. J. Colacot, E. S. Gore, A. Kuber, Organometallics 2002, 21, 3301;
- 12cC. A. Parrish, S. L. Buchwald, J. Org. Chem. 2001, 66, 3820;
- 12dK. Inada, N. Miyaura, Tetrahedron 2000, 56, 8661;
- 12eY. Uozumi, H. Danjo, T. Hayashi, J. Org. Chem. 1999, 64, 3384;
- 12fI. Fenger, C. Le Drian, Tetrahedron Lett. 1998, 39, 4287;
- 12gS.-B. Jang, Tetrahedron Lett. 1997, 38, 1793.
- 13For soluble polymer-supported phosphines for Suzuki coupling, see:
- 13aQ.-S. Hu, Y. Lu, Z.-Y. Tang, H.-B. Yu, J. Am. Chem. Soc. 2003, 125, 2856;
- 13bA. Datta, K. Ebert, H. Plenio, Organometallics 2003, 22, 4685. Although not used in Suzuki reactions, a soluble poly(arylenediphosphine) has been used in other Pd-catalyzed reactions:
- 13cT. Kanbara, S. Takase, R. Hayashi, S. Kagaya, K. Hasegawa, T. Yamamoto, J. Polym. Sci. Part A 2002, 40, 2637.
- 14There are two possible explanations for the low catalytic activity observed with 2. One possibility is that steric bulk around the phosphorus center in 2 hinders coordination to Pd. A second possibility is that the high phosphorus density in 2 leads to multidentate coordination to Pd, thus rendering it inaccessible to substrates and inactive.
- 15Homopolymers have been prepared from a variety of substituted monomers (RPCR2): M. Yam, C.-W. Tsang, K. Noonan, J. Kingsley, B. O. Patrick, D. P. Gates, unpublished results.