The Strongest Isolable Acid†
This work was supported by NSF grant CHE-0095206 and NIH grant GM 23851.
Graphical Abstract
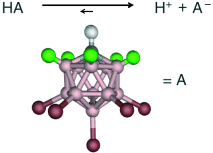
Measure for measure: Several measures indicate that carborane acids of the type H(CHB11R5X6) (see scheme, green R, red X, gray H, pink B) for R=H, Cl and X=Cl, Br, I are the strongest pure Brønsted acids. Based on NMR and IR spectroscopy, H(CHB11Cl11) can lay claim to be the strongest isolable acid presently known.
Acids based on carborane anions as conjugate bases (Figure 1) are a new class of Brønsted (protic) acids, notable for their “strong yet gentle” qualities.1 For example, whereas conventional strong acids (e.g. H2SO4) and superacids (e.g. HFSO3/SbF5) decompose fullerenes even at low temperatures, the carborane acid H(CHB11H5Cl6) cleanly protonates C60 at room temperature to give the isolable salt [HC60][CHB11H5Cl6]. We now show that carborane acids are the strongest isolable (Lewis-free) Brønsted acids presently known.
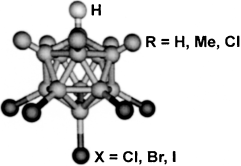
The [CHB11R5X6]− carborane ions.
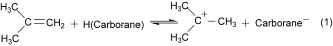 (1)
(1)Since mixed Lewis/Brønsted superacid media such as “Magic Acid” (HFSO3/SbF5) having H0 somewhere in the range −17 to −27 are used to stabilize tert-butyl cation,5 it is apparent that carborane acids have intrinsic Brønsted acidities comparable to those only found previously in Lewis/Brønsted acid mixtures.
The acidities of carborane acids cannot be measured in the conventional manner of an H0 Hammett acidity function because carborane acids are solids not liquids. They sublime under vacuum, or melt at atmospheric pressure, at temperatures well above 150 °C. Gas-phase acidities have been calculated for carborane acids, which rank them the strongest of any known isolable acid,6 but it is presently not possible to translate gas-phase ΔG data into measures of condensed-phase acidity.
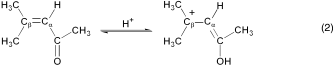 (2)
(2)13C NMR spectroscopy data for 0.15 M solutions of various acids and 0.10 M mesityl oxide were obtained at room temperature and are given in Table 1. The choice of oxyacids for comparison to carborane acids is limited by their availability in pure form and the need for miscibility or dissolution in liquid SO2. Nevertheless, it is immediately evident from their high chemical shift values that, as a class, carborane acids are stronger than conventional oxyacids. They outrank fluorosulfuric acid, the strongest known pure Brønsted acid on the H0 acidity scale (−15.1), as well as triflic acid (H0=−14.1). A strict correlation between Δδ values in liquid SO2 and the H0 values of neat acids is not expected because acidity is a solvation-dependent phenomenon that changes as the medium changes. Nevertheless, for the limited data set available, the ranking of acid strength by Δδ values follows the H0 order (Table 1).
|
Acid |
Δδ (13C) [ppm] |
H0 |
|---|---|---|
|
H(CHB11Cl11) |
84.0±0.1 |
[a] |
|
H(CHB11H5Cl6) |
83.8±0.1 |
[a] |
|
H(CHB11H5Br6) |
83.8±0.1 |
[a] |
|
H(CHB11H5I6) |
83.3±0.1 |
[a] |
|
FSO3H |
73.8±0.5 |
−15.1 |
|
CF3SO3H |
72.9±0.4 |
−14.1 |
|
HN(SO2CF3)2 |
72.0±0.4 |
[a] |
|
H2SO4 |
64.3±3.1[b] |
−12.1 |
|
Unprotonated mesityl oxide |
32.4±0.1 |
- [a] H0 values unavailable because acids are solids, not liquids. [b] Incomplete miscibility of H2SO4 in liquid SO2 leads to higher error limits and possible underestimate of Δδ.
It is also evident from the data of Table 1 that whereas oxyacids only partially protonate mesityl oxide, carborane acids are strong enough to move Equation (2) completely to the right hand side, that is, their acidities are leveled. The Δδ value maximizes at δ=84.0 ppm. Though close to the limits of discrimination, the hexa-iodo carborane acid, [H(CHB11H5I6)] with a value of δ=83.3 ppm, appears to be perceptibly weaker than its chloro and bromo counterparts.
To discriminate between the acidities of the different carborane acids, we have developed a new, qualitative ranking of acidity based on the νNH frequencies of the ammonium salts of their conjugate base anions. The ranking is based on the influence of the anion A− on the NH bond in a contact ion pair (Figure 2).
Ammonium salt contact ion pair.
The weaker the interaction of the base A−, the stronger the NH bonding, and the higher the νNH frequency.8 Since νNH frequencies (>3000 cm−1) are energetically far removed from νHA frequencies (<300 cm−1), vibrational coupling or mass differences in A− will have an insignificant effect on νNH, that is, νNH frequencies should be a good measure of NH bond strength. Since hydrogen bonding is a predominantly electrostatic phenomenon,9 NH bond strength should correlate strongly with A− basicity, that is, with HA acidity. νNH frequencies will decrease with increasing A− basicity, correlating with HA acidity. In a constant solvation environment within a series of isostructural anions, the correlation should be quite direct. Although the direct correlation of acidity with IR stretching frequency has been questioned for solid surfaces,10–12 acidity rankings based on IR data have been shown to have excellent validity within a number of classes of discrete compounds, particularly when structurally related.13–17
Tri-n-octylammonium salts of carborane anions were prepared by silver-salt metathesis reactions and characterized as detailed in the Supporting Information. Octyl groups in the cation were chosen to provide good solubility of [n-Oct3NH][carborane] ion pairs in CCl4 solution. To avoid possible effects from aggregation, νNH frequencies were measured in dilute solution where there was no concentration dependence (Table 2). The strong dependence of νNH on the nature of the anion indicates that contact ion pairs are formed. In the more polar solvent 1,2-dichloroethane, the rank order is the same but some salts showed a second band of invariable frequency (3179±1 cm−1) which is assigned to solvent-separated ion pairs. For the corresponding deuterated [Oct3ND]+ salts the νND/νNH ratio was 1.339±0.004, indicating the absence of anharmonicity effects or Fermi resonance.
 NH frequencies of tri-n-octylammonium salts in CCl4.
NH frequencies of tri-n-octylammonium salts in CCl4.|
Anion |
νNH [±1 cm−1] |
Δν [cm−1] |
|---|---|---|
|
[CHB11Cl11]− [a] |
3163 |
0 |
|
[CHB11Me11]− |
3156 |
7 |
|
[CHB11H5Cl6]− [a] |
3148 |
15 |
|
[CoIII(C2B9H8Cl3)2]− |
3145 |
18 |
|
[CHB11Me5Cl6]− |
3143 |
20 |
|
[CHB11H11]− |
3129 |
34 |
|
[CHB11H5Br6]− [a] |
3125 |
38 |
|
[CHB11Me5Br6]− |
3120 |
43 |
|
[CHB11H5I6]− [a] |
3097 |
66 |
|
[CHB11Me5I6]− |
3091 |
72 |
- [a] Denotes presently known isolable acids.
The νNH ranking brings out a number of features: a) For each of the pairs of hexahalo carboranes [CHB11H5X6]− (X=Cl, Br, I,) and their pentamethylated counterparts [CHB11Me5X6]−,18 the difference in νNH values is 5 or 6 cm−1. This consistency gives confidence in the scale, suggesting that the CCl4 solvation environment remains constant over the series and that mass differences and steric effects have negligible influence. It is evident that small differences in anion basicity can be detected quite accurately and the scale should be useful for a much wider range of weakly coordinating anions. b) The ranking of the hexahalo carborane acids, H(CHB11H5Cl6)>H(CHB11H5Br6)>H(CHB11H5I6), makes sense on the basis of electronegativity and polarizability considerations for the halogen substituents. It is consistent with conceptually related deductions drawn recently from νCH frequencies in benzenium ion salts having the same series of carborane counterions.3 c) The top ranking (i.e. least basic) carborane anion measured to date is the undecachlorinated ion, [CHB11Cl11]−,19 which leads to the prediction that its conjugate acid will be the strongest. As detailed in the Supporting Information, H(CHB11Cl11) can be prepared in a similar manner to the hexahalo carborane acids as a sublimable, analytically-pure, colorless solid by the reaction of Et3Si(CHB11Cl11) with anhydrous HCl. Thus, H(CHB11Cl11) can lay claim to be the strongest isolable acid presently known.
Carborane acids are strong acids because their anions are exceptionally weak bases. This arises from the large size of the anions, the delocalized nature of the negative charge in the [CB11]− cluster, and the shield of halide substituents. However, there is an important additional necessity for preparing such a strong acid, namely, the anion must be exceptionally inert from a chemical point of view. The inertness of carborane anions arises from σ aromaticity within the [CB11]− icosahedral cage, a notable feature of boron cluster chemistry. The [CB11]− framework resists chemical disruption to a truly exceptional degree. There are anions that probably have lower basicity than carboranes, for example, organofluoro anions, such as [B(C6F5)4]−,20 but their conjugate acids cannot be prepared because the anion decomposes at high acidity.21 This situation is mirrored in that traditional fluoroacids, commonly written as HBF4, HPF6, HSbF6 etc., are also nonexistent compounds. They are all unstable with respect to formation of HF and the corresponding Lewis acid. The superacid commonly written “HSbF6” is actually a mixture of [H(HF)x][SbF6⋅nSbF5] ions in HF/SbF5 solvent and cannot be isolated as a pure Brønsted acid. Thus, present limits to acid strength are seen to be more dependent on considerations of anion stability than basicity, although low basicity is clearly required.
An important and potentially very useful feature of carborane acids is that their high protic acidity is expressed in the absence of an added Lewis acid. The presence of Lewis acids in traditional superacids (e.g. HFSO3/SbF5) can interfere with protonation chemistry and limit their usefulness in two identifiable ways. As alluded to earlier for C60, Lewis acids (particularly SbF5) can supply oxidizing equivalents and halide nucleophiles that together, conspire to decompose many protonated unsaturated molecules. A more subtle effect arises from the likely formation of Lewis acid/base complexes when substrates are investigated in mixed Brønsted/Lewis superacids. If a substrate forms an adduct with a Lewis acid, its basicity will be lowered and it will become much more difficult to protonate. This phenomenon can be called “basicity suppression”. It suggests that the basicities of weakly basic molecules may have been significantly underestimated, and makes the pursuit of even stronger pure Brønsted acids an important endeavor.
Carborane acids may lead not only to the isolation and stabilization of protonated species hitherto unattainable, but also to a reassessment of the intrinsic basicity of weakly basic molecules. Experiments to test this hypothesis are underway.