Highly Active, Binary Catalyst Systems for the Alternating Copolymerization of CO2 and Epoxides under Mild Conditions†
Gratitude is expressed to the National Science Foundation of China (NSFC) program (Grant 20 204 002) and the Science Foundation of Liaoning Province (Grant 20 031 074) for financial support.
Graphical Abstract
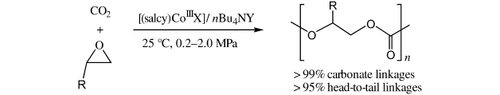
Excellent activity and selectivity in the copolymerization of CO2 with epoxides at extremely mild temperature and pressure are observed in the presence of binary nucleophile–electrophile catalyst systems based on chiral [(salcy)CoIIIX] complexes and quaternary ammonium salts (see scheme). Completely alternating copolymers are obtained with >95 % head-to-tail linkages.
Chemical fixation of CO2 is of great interest with respect to the development of truly environmentally benign processes, as there are many possibilities for CO2 to be used as a safe and cheap C1 feedstock in organic synthesis.1 One of the most promising reactions in this area is the alternating copolymerization of CO2 and epoxides to polycarbonates,2 which was first reported by Inoue et al.3 These polycarbonates not only exhibit interesting material properties, but also have the additional environmental advantages resulting from their biodegradability.4 In recent decades, numerous catalyst systems have been developed for this transformation.5 Although the advances have been significant, high catalyst loading, elevated CO2 pressure, and long reaction time are usually prerequisites for obtaining appreciable amounts of polymer, which are often not perfectly alternating and exhibit broad molecular-weight distributions. Recently, several efficient catalyst systems for the copolymerization of CO2 and alicyclic epoxides such as cyclohexene oxide have been reported to offer significant advantages over traditional heterogeneous catalysts.6–12 Unfortunately, these catalysts do not readily polymerize CO2 and aliphatic epoxides such as propylene oxide (PO). Recently, Coates and co-workers13 reported the first highly active zinc β-diiminate catalysts bearing electron-withdrawing groups for the synthesis of poly(propylene carbonate) (PPC) with carbonate linkages up to 99 %, but along with the formation of cyclic propylene carbonate (PC; 7–25 %). A simple decrease in temperature and increase in CO2 pressure suppressed the formation of PC and increased the selectivity for PPC. A similar effect was reported by Darensbourg and co-workers, who used chiral salen chromium chloride, alone or in conjunction with N-methylimidazole as a catalyst, for this reaction.14 Herein, we report the use of a highly active binary catalyst system, which consists of a chiral cobalt complex [(salcy)CoIIIX] and a quaternary ammonium salt (nBu4NY) for completely alternating copolymerization of CO2 and aliphatic epoxides under extremely mild conditions.
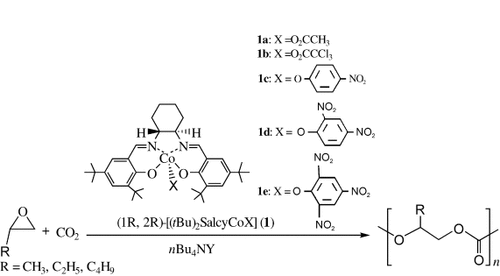
Pursuant to our own efforts toward the development of highly efficient catalysts for coupling CO2 with epoxides under mild conditions,15 we became interested in the possibility of developing catalyst systems for the direct synthesis of optically active cyclic carbonates from racemic epoxides, which are inexpensive or easily accessible from inexpensive commercial starting materials. Based on the fact that chiral [(salcy)CoIII] complexes (Jacobsen catalyst) were shown to be highly efficient and enantioselective catalysts for the hydrolytic kinetic resolution of terminal epoxides,16 we recently designed a bifunctional nucleophile–electrophile catalyst system consisting of chiral [(salcy)CoIII] complexes and quaternary ammonium salts for the direct synthesis of optically active cyclic carbonates from racemic epoxides.17 In this system, the chiral [(salcy)CoIII] complex was proposed to serve as an electrophile for selectively complexing one enantiomer of the racemic epoxides, and the anion of the quaternary ammonium salt serves as a nucleophile. The regioselective attack of a nucleophile or activated CO2 at the coordinated epoxide on the less substituted carbon atom leads to enantioselective ring opening of the epoxide with subsequent formation of the chiral cyclic carbonates through intermolecular cyclic elimination (Scheme 1). We were delighted to find that the solvent-free reaction of racemic PO (0.2 mol) with CO2 in the presence of [(salcy)CoIII(O2CCCl3)] (1 b; 0.05 mol %) as a chiral electrophile in conjunction with nBu4NBr (0.05 mol %) as nucleophile proceeded within 3 h at room temperature and 2.0 MPa pressure to afford a mixture of unconverted PO and PC with moderate enantioselectivity (Table 1, entry 2), along with a small quantity of PPC. To our surprise, the resulting PPC shows >99 % carbonate linkage in the 1H NMR spectrum. Further investigation showed that simple changes in the axial group X of chiral [(salcy)CoIIIX] and the anion Y of nBu4NY drastically affect the PPC/PC selectivity (Table 1, entries 1–8). It seems that the use of a cobalt complex with an electron-withdrawing axial group X and a quaternary ammonium salt, whose anion exhibits poor leaving ability, increases the selectivity for PPC. For example, in the presence of nBu4NBr cocatalyst, a change in the axial group X of chiral [(salcy)CoIIIX] from acetate to dinitrophenol resulted in an increase in the selectivity of PPC from 3 to 78 %. On the other hand, with complex 1 d as catalyst, a change in the anion of cocatalyst nBu4NY from Br− to Cl− or CH3COO− resulted in an increase in the selectivity for PPC from 78 to 99 %. The results may be explained in terms of greater advantages for producing linear polycarbonates by successive insertion of CO2/epoxide towards the M–O bond as compared with the formation of cyclic carbonates by intermolecular cyclic elimination (Scheme 1) in the catalyst systems that consist of a strong electrophile in conjunction with a nucleophile with poor leaving ability. Notably, these binary catalyst systems based on chiral [{(1R,2R)-(tBu)2salcy}CoX] complexes all preferentially consume (S)-PO over (R)-PO with Krel=2.8–3.5. Notably, these cobalt complexes or quaternary ammonium salts by themselves show no (or only very low) catalytic activity under the conditions employed. Interestingly, the binary catalyst systems are inactive for the homopolymerization of PO over 1 day.
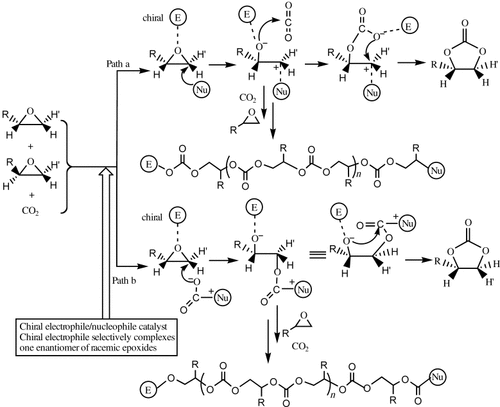
A bifunctional nucleophile–electrophile catalyst system for the direct synthesis of enantiomerically pure cyclic carbonates or linear polycarbonates from racemic epoxides and CO2.
- [a] The reaction was carried out with neat epoxide (14 mL, 200 mmol; catalyst/co-catalyst/epoxide=1:1:2000) at 25 °C, unless otherwise noted. [b] Turnover frequency of epoxide to products (polycarbonate and cyclic carbonate). [c] Determined by using 1H NMR spectroscopy. [d] Determined by means of gel permeation chromatography in THF at 35 °C, calibrated with polystyrene standards. [e] Krel=ln[1-c(1+ee)]/ln[1-c(1-ee)]; c=conversion, ee=enantiomeric excess of unconverted epoxide. [f] Not applicable. [g] 40 °C. [h] 1,2-Butene oxide. [i] 1,2-hexene oxide.
A significant drawback in the use of CO2 as a reagent in organic synthesis is the potential danger associated with operating at high temperatures and pressures. Prior to the present work, Coates et al. reported the first discrete cobalt complexes alone as catalysts for the copolymerization of CO2 and PO, but these complexes are inactive at low CO2 pressures.18 We were gratified to discover that the binary catalyst system can operate very efficiently at low temperatures and with CO2 at atmospheric pressure. The highest activity occurs at 1.0–2.0 MPa and is maintained at 50 % of the optimum TOF at only 0.2 MPa. An increase in the pressure beyond the optimal range results in a dramatic decrease in activity. Interestingly, the variation of CO2 pressure does not lead to an observable decrease in selectivity for PPC formation. In contrast, in the presence of [(bdi)ZnOAc] catalyst (bdi=β-diiminate),13 an increase in the pressure of CO2 is beneficial for suppressing PC formation and effectively increases the selectivity for polymer while moderately decreasing the catalyst activity for PPC formation. Surprisingly, a change in the reaction temperature from 25 to 40 °C, only significantly affects the activity of the catalyst, rather than the selectivity for PPC formation (Table 1, entry 16).
All the isolated polymers have narrow molecular-weight distributions, consistent with controlled polymerization; however, the Mn values are not close to the expected values, which indicates the existence of chain transfer during the reaction. The 1H NMR spectra of all PPCs produced show >99 % carbonate linkages, and the 13C NMR spectra show that these polymers have an unprecedented head-to-tail content of >95 % (Figure 1). Such a microstructure is consistent with a highly stereoselective ring opening of PO, and is in contrast to polymers prepared in the presence of zinc glutarate19 (∼60 % head-to-tail linkages) and [(bdi)ZnOAc] (∼54 % head-to-tail linkages). Also, the catalyst system was found to be applicable to other aliphatic epoxides, providing the corresponding polycarbonates with >99 % carbonate linkages (Table 1, entries 17 and 18).
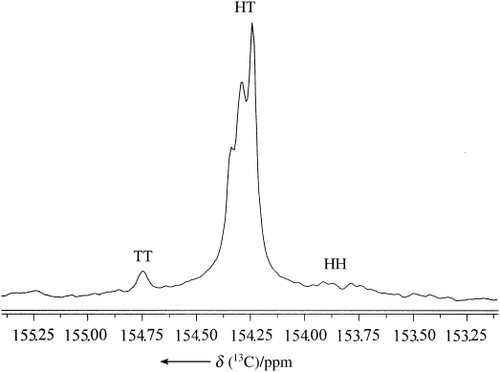
The carbonyl region of the 13C NMR spectrum of a representative sample of poly(propylene carbonate) (PPC).
In summary, chiral [(salcy)CoIIIX] in conjunction with a quaternary ammonium salt is a highly efficient catalyst for the copolymerization of CO2 with epoxides under extremely mild temperatures and pressures to afford the corresponding completely alternating copolymers with >95 % head-to-tail linkages. To our knowledge, these binary catalyst systems exhibit the highest reported activities for PO/CO2 copolymerization. Furthermore, they offer unprecedented opportunities for controlling the regio- and stereochemistry of copolymerization. Further studies are focused on elucidating the catalytic mechanism and developing new bifunctional nucleophile–electrophile catalyst systems that exhibit higher regio- and stereoselectivity for epoxide ring opening in CO2/epoxide copolymerization.