Mixed Helices—A General Folding Pattern in Homologous Peptides?†
Carsten Baldauf Dipl.-Biochem.
Institut für Biochemie, Universität Leipzig, Brüderstrasse 34, 04103 Leipzig, Germany, Fax: (+49) 341-973-6998
Search for more papers by this authorRobert Günther Dr.
Institut für Biochemie, Universität Leipzig, Brüderstrasse 34, 04103 Leipzig, Germany, Fax: (+49) 341-973-6998
Search for more papers by this authorHans-Jörg Hofmann Prof.
Institut für Biochemie, Universität Leipzig, Brüderstrasse 34, 04103 Leipzig, Germany, Fax: (+49) 341-973-6998
Search for more papers by this authorCarsten Baldauf Dipl.-Biochem.
Institut für Biochemie, Universität Leipzig, Brüderstrasse 34, 04103 Leipzig, Germany, Fax: (+49) 341-973-6998
Search for more papers by this authorRobert Günther Dr.
Institut für Biochemie, Universität Leipzig, Brüderstrasse 34, 04103 Leipzig, Germany, Fax: (+49) 341-973-6998
Search for more papers by this authorHans-Jörg Hofmann Prof.
Institut für Biochemie, Universität Leipzig, Brüderstrasse 34, 04103 Leipzig, Germany, Fax: (+49) 341-973-6998
Search for more papers by this authorThis work was supported by the Deutsche Forschungsgemeinschaft (Project HO2346/1 “Sekundärstrukturbildung in Peptiden mit nicht-proteinogenen Aminosäuren” and SFB 610 “Proteinzustände mit zellbiologischer und medizinischer Relevanz”) and the Fonds der Chemischen Industrie.
Graphical Abstract
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2004/z53249_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1D. J. Hill, M. J. Mio, R. B. Prince, T. S. Hughes, J. S. Moore, Chem. Rev. 2001, 101, 3893.
- 2S. H. Gellman, Acc. Chem. Res. 1998, 31, 173.
- 3
- 3aR. P. Cheng, S. H. Gellman, W. F. DeGrado, Chem. Rev. 2001, 101, 3219;
- 3bD. Seebach, J. L. Matthews, Chem. Commun. 1997, 2015;
- 3cW. F. DeGrado, J. P. Schneider, Y. Hamuro, J. Pept. Res. 1999, 54, 206.
- 4
- 4aT. Hintermann, K. Gademann, B. Jaun, D. Seebach, Helv. Chim. Acta 1998, 81, 983;
- 4bS. Hanessian, X. Luo, R. Schaum, S. Michnick, J. Am. Chem. Soc. 1998, 120, 8569;
- 4cD. D. Long, N. L. Hungerford, M. D. Smith, D. E. A. Brittain, D. G. Marquess, T. D. W. Claridge, G. W. J. Fleet, Tetrahedron Lett. 1999, 40, 2195.
- 5
- 5aD. Seebach, K. Gademann, J. V. Schreiber, J. L. Matthews, T. Hintermann, B. Jaun, Helv. Chim. Acta 1997, 80, 2033;
- 5bM. Rueping, J. V. Schreiber, G. Lelais, B. Jaun, D. Seebach, Helv. Chim. Acta 2002, 85, 2577.
- 6
- 6aS. A. W. Gruner, B. Truffault, G. Voll, E. Locardi, M. Stöckle, H. Kessler, Chem. Eur. J. 2002, 8, 4366;
- 6bG. V. M. Sharma, K. R. Reddy, P. R. Krishna, A. R. Sankar, K. Narsimulu, S. K. Kumar, P. Jayaprakash, B. Jagannadh, A. C. Kunwar, J. Am. Chem. Soc. 2003, 125, 13 670.
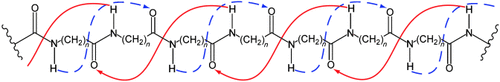
- 7Variation of the backbone torsion angles based on the alternation conditions for mixed helices: α-peptides: φ, ψ in steps of 30°; β-peptides: φ, θ, ψ in steps of 30°; γ-peptides: φ, θ, ζ ,ψ in steps of 60°; δ-peptides: φ and ψ in steps of 60°, θ, ζ, and ρ in steps of 120°; changes of ±15° were allowed for the torsion angle ω=180° of the trans-peptide bonds.
- 8aGaussian 98 (Revision A.11.3), M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, C. Gonzalez, M. Challacombe, P. M. W. Gill, B. G. Johnson, W. Chen, M. W. Wong, J. L. Andres, M. Head-Gordon, E. S. Replogle, J. A. Pople, Gaussian, Inc., Pittsburgh, PA, 1998;
- 8bGamess (Version: 20 JUN 2002 (R2)) M. W. Schmidt, K. K. Baldridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, S. Koseki, N. Matsunaga, K. A. Nguyen, S. J. Su, T. L. Windus, M. Dupuis, J. A. Montgomery, J. Comput. Chem. 1993, 14, 1347.
- 9
- 9aT. Head-Gordon, M. Head-Gordon, M. J. Frisch, C. L. Brooks III, J. A. Pople, J. Am. Chem. Soc. 1991, 113, 5989;
- 9bG. Endredi, A. Perczel, O. Farkas, M. A. McAllister, G. I. Csonka, J. Ladik, I. G. Csizmadia, J. Mol. Struct. 1997, 391, 15;
- 9cK. Möhle, M. Gussmann, A. Rost, R. Cimiraglia, H.-J. Hofmann, J. Phys. Chem. A 1997, 101, 8571.
- 10
- 10aF. A. Momany, R. Rone, J. Comput. Chem. 1992, 13, 888;
- 10bF. A. Momany, R. Rone, H. Kunz, R. F. Frey, S. Q. Newton, L. Schäfer, J. Mol. Struct. 1993, 286, 1.
- 11
- 11aD. Seebach, M. Overhand, F. N. M. Kühnle, B. Martinoni, L. Oberer, U. Hommel, H. Widmer, Helv. Chim. Acta 1996, 79, 913;
- 11bD. H. Appella, L. A. Christianson, D. A. Klein, D. R. Powell, X. Huang, J. J. Barchi, Jr.,S. H. Gellman, Nature 1997, 387, 381;
- 11cK. Möhle, R. Günther, M. Thormann, N. Sewald, H.-J. Hofmann, Biopolymers 1999, 50, 167;
10.1002/(SICI)1097-0282(199908)50:2<167::AID-BIP6>3.0.CO;2-M CAS PubMed Web of Science® Google Scholar
- 11dY.-D. Wu, D.-P. Wang, J. Am. Chem. Soc. 1998, 120, 13 485;
- 11eX. Daura, K. Gademann, B. Jaun, D. Seebach, W. F. van Gunsteren, A. E. Mark, Angew. Chem. 1999, 111, 249;
10.1002/(SICI)1521-3757(19990115)111:1/2<249::AID-ANGE249>3.0.CO;2-B Google ScholarAngew. Chem. Int. Ed. 1999, 38, 236;10.1002/(SICI)1521-3773(19990115)38:1/2<236::AID-ANIE236>3.0.CO;2-M CAS Web of Science® Google Scholar
- 11fR. Günther, H.-J. Hofmann, K. Kuczera, J. Phys. Chem. B 2001, 105, 5559.
- 12C. Baldauf, R. Günther, H.-J. Hofmann, Helv. Chim. Acta 2003, 86, 2573.
- 13
- 13aY.-D. Wu, D. P. Wang, J. Am. Chem. Soc. 1999, 121, 9352;
- 13bT. A. Martinek, F. Fülöp, Eur. J. Biochem. 2003, 270, 3657.