Self-Assembled Aggregates of IgGs as Templates for the Growth of Clusters of Gold Nanoparticles†
Jerry Yang Prof.
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, MA 02138, USA, Fax (+1) 617-495-9857
Search for more papers by this authorMichael Mayer Dr.
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, MA 02138, USA, Fax (+1) 617-495-9857
Search for more papers by this authorJennah K. Kriebel
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, MA 02138, USA, Fax (+1) 617-495-9857
Search for more papers by this authorPiotr Garstecki Dr.
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, MA 02138, USA, Fax (+1) 617-495-9857
Search for more papers by this authorGeorge M. Whitesides Prof.
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, MA 02138, USA, Fax (+1) 617-495-9857
Search for more papers by this authorJerry Yang Prof.
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, MA 02138, USA, Fax (+1) 617-495-9857
Search for more papers by this authorMichael Mayer Dr.
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, MA 02138, USA, Fax (+1) 617-495-9857
Search for more papers by this authorJennah K. Kriebel
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, MA 02138, USA, Fax (+1) 617-495-9857
Search for more papers by this authorPiotr Garstecki Dr.
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, MA 02138, USA, Fax (+1) 617-495-9857
Search for more papers by this authorGeorge M. Whitesides Prof.
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, MA 02138, USA, Fax (+1) 617-495-9857
Search for more papers by this authorThis work was supported by the NIH (Grant GM30367). M.M. acknowledges the Swiss National Science Foundation for a postdoctoral fellowship. J.K. acknowledges support from the NDSEG for a predoctoral fellowship. P.G acknowledges the Foundation for Polish Science for a postdoctoral fellowship. We thank Dr. Declan Ryan for helpful discussions. IgGs=Immunoglobulin Gs.
Graphical Abstract
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2004/z53161_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aD. V. Goia, E. Matijevic, New J. Chem. 1998, 22, 1203;
- 1bD. L. Feldheim, C. A. Foss, Metal Nanoparticles: Synthesis, Characterization, and applications, Marcel Dekker, New York, 2002;
- 1cA. Kiriy, S. Minko, G. Gorodyska, M. Stamm, Nano Lett. 2002, 2, 881;
- 1dT. Scheibel, R. Parthasarathy, G. Sawicki, X. M. Lin, H. Jaeger, S. L. Lindquist, Proc. Natl. Acad. Sci. USA 2003, 100, 4527;
- 1eA. J. Haes, R. P. Van Duyne, J. Am. Chem. Soc. 2002, 124, 10 596.
- 2K. J. Klabunde, Nanoscale materials in Chemistry, Wiley, New York, 2001.
10.1002/0471220620 Google Scholar
- 3
- 3aW. P. McConnell, J. P. Novak, L. C. Brousseau, R. R. Fuierer, R. C. Tenent, D. L. Feldheim, J. Phys. Chem. B 2000, 104, 8925;
- 3bC. A. Mirkin, R. L. Letsinger, R. C. Mucic, J. J. Storhoff, Nature 1996, 382, 607;
- 3cW. Shenton, S. A. Davis, S. Mann, Adv. Mater. 1999, 11, 449.
- 4
- 4aQ. Wang, T. Lin, L. Tang, J. E. Johnson, M. G. Finn, Angew. Chem. 2002, 114, 477;
Angew. Chem. Int. Ed. 2002, 41, 459;
10.1002/1521-3773(20020201)41:3<459::AID-ANIE459>3.0.CO;2-O CAS PubMed Web of Science® Google Scholar
- 4bQ. Wang, T. Lin, J. E. Johnson, M. G. Finn, Chem. Biol. 2002, 9, 813;
- 4cQ. Wang, E. Kaltgrad, T. Lin, J. E. Johnson, M. G. Finn, Chem. Biol. 2002, 9, 805;
- 4dK. Keren, M. Krueger, R. Gilad, G. Ben-Yoseph, U. Sivan, E. Braun, Science 2002, 297, 72;
- 4eC. Joachim, J. K. Gimzewski, A. Aviram, Nature 2000, 408, 541;
- 4fW. Shenton, D. Pum, U. B. Sleytr, S. Mann, Nature 1997, 389, 585;
- 4gC. Mao, C. E. Flynn, A. Hayhurst, R. Sweeney, J. Qi, G. Georgiou, B. Iverson, A. M. Belcher, Proc. Natl. Acad. Sci. USA 2003, 100, 6946;
- 4hC. M. Niemeyer, W. Burger, J. Peplies, Angew. Chem. 1998, 110, 2391;
10.1002/(SICI)1521-3757(19980817)110:16<2391::AID-ANGE2391>3.0.CO;2-X Google ScholarAngew. Chem. Int. Ed. 1998, 37, 2265;10.1002/(SICI)1521-3773(19980904)37:16<2265::AID-ANIE2265>3.0.CO;2-F CAS PubMed Web of Science® Google Scholar
- 4iS. Mann, W. Shenton, M. Li, S. Connolly, D. Fitzmaurice, Adv. Mater. 2000, 12, 147.
- 5A. P. Alivisatos, K. P. Johnsson, X. Peng, T. E. Wilson, C. J. Loweth, M. P. Bruchez, P. G. Schultz, Nature 1996, 382, 609; Professor Matthew B. Francis, personal communication.
- 6E. Dujardin, S. Mann, Adv. Mater. 2002, 14, 775.
- 7
- 7aL. C. Brousseau, J. P. Novak, S. M. Marinakos, D. L. Feldheim, Adv. Mater. 1999, 11, 447;
- 7bJ. P. Novak, L. C. Brousseau, F. W. Vance, R. C. Johnson, B. I. Lemon, J. T. Hupp, D. L. Feldheim, J. Am. Chem. Soc. 2000, 122, 12 029;
- 7cJ. P. Novak, D. L. Feldheim, J. Am. Chem. Soc. 2000, 122, 3979.
- 8
- 8aM. Mertig, L. C. Ciacchi, R. Seidel, W. Pompe, A. De Vita, Nano Lett. 2002, 2, 841;
- 8bS. Minko, A. Kiriy, G. Gorodyska, M. Stamm, J. Am. Chem. Soc. 2002, 124, 10 192.
- 9E. E. Simanek, J. P. Mathias, C. T. Seto, D. Chin, M. Mammen, D. M. Gordon, G. M. Whitesides, Acc. Chem. Res. 1995, 28, 37.
- 10Green,[11] Valentine,[12] and others[13] described the spontaneous formation of linear and cyclic nanoscale complexes between polyclonal IgGs[14] (or IgEs) and divalent antigens.
- 11aN. M. Green, Adv. Immunol. 1969, 11, 1;
- 11bR. C. Valentine, N. M. Green, J. Mol. Biol. 1967, 27, 615.
- 12aR. C. Valentine in Nobel Symposium, Vol. 3, Wiley-Interscience, New York, 1967, p. 251;
- 12bR. C. Valentine in Proceedings of the European Regional Conference on Electron Microscopy, Vol. 2, Rome, 1969, p. 3.
- 13aM. Mammen, S.-K. Choi, G. M. Whitesides, Angew. Chem. 1998, 110, 2909;
10.1002/(SICI)1521-3757(19981016)110:20<2908::AID-ANGE2908>3.0.CO;2-2 Google ScholarAngew. Chem. Int. Ed. 1998, 37, 2755;10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3 CAS Web of Science® Google Scholar
- 13bB. Baird, B. Goldstein, R. Posner, K. Subramanian, B. Hashemi, D. Holowka, J. Cell. Biochem. 1994, Suppl. 18C, 241;
- 13cM. Dembo, B. Goldstein, A. K. Sobotka, L. M. Lichtenstein, J. Immunol. 1978, 121, 354;
- 13dJ. W. Erickson, R. G. Posner, B. Goldstein, D. Holowka, B. Baird, Biochemistry 1991, 30, 2357;
- 13eR. G. Posner, J. W. Erickson, D. Holowka, B. Baird, B. Goldstein, Biochemistry 1991, 30, 2348;
- 13fR. Schweitzerstenner, A. Licht, I. Pecht, Biophys. J. 1992, 63, 563.
- 14IgGs are proteins that comprise four polypeptide chains joined by disulfide bonds to give a Y-shaped glycoprotein.[15] There are two antigen-binding Fab fragments and one crystallizable Fc fragment, which make up the three arms of the Y in the IgG molecules. The Fab arms join the Fc region at a flexible hinge region; the angle between the two Fab regions can vary from 40 to over 180 degrees;[16] the flexibility at the hinge allows for the generation of several different sizes and shapes of cyclic oligomers of IgGs. The length of the Fab and Fc regions varies from organism to organism, but on average the Fab fragment is about 12 nm long and the Fc fragment is about 11 nm long.[10a,17]
- 15C. A. Janeway, P. Travers, M. Walport, J. D. Capra, Immunobiology, 4th ed., Garland, New York, 1999.
- 16M. Gerstein, A. M. Lesk, C. Chothia, Biochemistry 1994, 33, 6739.
- 17D. M. Crothers, H. Metzger, Immunochemistry 1972, 9, 341.
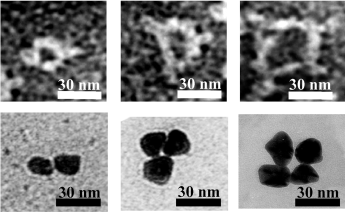
- 18We will report the details of these studies in solution elsewhere. The supporting information includes a representative size-exclusion HPLC chromatogram illustrating the conversion of IgGs to aggregated species.
- 19We separated these aggregates from the non-aggregated protein by FPLC (data not shown). Images of gold nanoparticles grown from a mixture of these purified aggregates appeared indistinguishable from images of particles grown from aggregates of IgGs that were not purified by FPLC.
- 20C. Branden, J. Tooze, Introduction to Protein Structure, 2nd ed., Garland, New York, 1999.
- 21H. W. Morehead, K. W. Talmadge, D. J. O′Shannessy, C. J. Siebert, J. Chromatogr. 1991, 587, 171.
- 22
- 22aA. Rambourg, C. P. Leblond, J. Cell Biol. 1967, 32, 27;
- 22bJ. J. Bozzola, L. D. Russell, Electron Microscopy, 2nd ed., Jones & Bartlett, Boston, 1999.
- 23Staining and TEM-imaging experiments of IgGs on Si3N4 substrates indicate that washing the surfaces in aqueous solutions does not completely remove protein from the substrates.
- 24We believe the difference in the fraction of aggregated IgGs in solution (≈73 % of the protein) and the fraction of clusters of nanoparticles grown from the carbohydrates on the IgGs (15±5 %) reflects the less than quantitative yield of the chemical reactions that generate the metal nanoparticles—that is, the oxidation of the carbohydrate units, the reduction of silver ions, and the electroless growth of gold.
- 25Adapted from J. Koolman, Color Atlas of Biochemistry Thieme, Stuttgart, 1996.