Synthesis of Versatile Chiral N,P Ligands Derived from Pyridine and Quinoline†
William J. Drury III Dr.
Department of Chemistry, University of Basel, St. Johanns-Ring 19, 4056 Basel, Switzerland, Fax: (+41) 61-267-1103
Search for more papers by this authorNicole Zimmermann Dr.
Department of Chemistry, University of Basel, St. Johanns-Ring 19, 4056 Basel, Switzerland, Fax: (+41) 61-267-1103
Search for more papers by this authorMartine Keenan Dr.
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorMasahiko Hayashi Dr.
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorStefan Kaiser Dipl.-Chem.
Department of Chemistry, University of Basel, St. Johanns-Ring 19, 4056 Basel, Switzerland, Fax: (+41) 61-267-1103
X-Ray analyses.
Search for more papers by this authorRichard Goddard Dr.
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
X-Ray analyses.
Search for more papers by this authorAndreas Pfaltz Prof. Dr.
Department of Chemistry, University of Basel, St. Johanns-Ring 19, 4056 Basel, Switzerland, Fax: (+41) 61-267-1103
Search for more papers by this authorWilliam J. Drury III Dr.
Department of Chemistry, University of Basel, St. Johanns-Ring 19, 4056 Basel, Switzerland, Fax: (+41) 61-267-1103
Search for more papers by this authorNicole Zimmermann Dr.
Department of Chemistry, University of Basel, St. Johanns-Ring 19, 4056 Basel, Switzerland, Fax: (+41) 61-267-1103
Search for more papers by this authorMartine Keenan Dr.
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorMasahiko Hayashi Dr.
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorStefan Kaiser Dipl.-Chem.
Department of Chemistry, University of Basel, St. Johanns-Ring 19, 4056 Basel, Switzerland, Fax: (+41) 61-267-1103
X-Ray analyses.
Search for more papers by this authorRichard Goddard Dr.
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
X-Ray analyses.
Search for more papers by this authorAndreas Pfaltz Prof. Dr.
Department of Chemistry, University of Basel, St. Johanns-Ring 19, 4056 Basel, Switzerland, Fax: (+41) 61-267-1103
Search for more papers by this authorFinancial support for this work from the American and Swiss National Science Foundations is gratefully acknowledged. N.Z. thanks the Fonds der Chemischen Industrie for a Kekulé fellowship. The authors kindly thank Dr. Thomas Lectka (The Johns Hopkins University) for helpful discussions.
Graphical Abstract
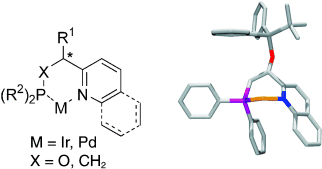
Potent transition-metal complexes for asymmetric catalysis are formed from readily accessible N,P ligands constructed from basic N-heteroaryl building blocks (see structure). Their simple assembly should not be misconstrued: they posses several handles by which to tune both steric and electronic parameters. The potential of these ligands is demonstrated by the high levels of enantioselection they induce in such divergent processes as asymmetric hydrogenation and the Heck reaction.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2004/z52755_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aG. Helmchen, A. Pfaltz, Acc. Chem. Res. 2000, 33, 336.
- 2
- 2aF. Menges, M. Neuburger, A. Pfaltz, Org. Lett. 2002, 4, 4713; for related ligands, see:
- 2bC. A. Busacca, D. Grossbach, R. C. So, E. M. O'Brien, E. M. Spinelli, Org. Lett. 2003, 5, 595.
- 3
- 3aJ. Blankenstein, A. Pfaltz, Angew. Chem. 2001, 113, 4577;
10.1002/1521-3757(20011203)113:23<4577::AID-ANGE4577>3.0.CO;2-P Google ScholarAngew. Chem. Int. Ed. 2001, 40, 4445;10.1002/1521-3773(20011203)40:23<4445::AID-ANIE4445>3.0.CO;2-V CAS PubMed Web of Science® Google Scholar
- 3bF. Menges, A. Pfaltz, Adv. Synth. Catal. 2002, 344, 40.
- 4A. Pfaltz, J. Blankenstein, R. Hilgraf, E. Hörmann, S. McIntyre, F. Menges, M. Schönleber, S. P. Smidt, B. Wüstenberg, N. Zimmermann, Adv. Synth. Catal. 2003, 345, 33.
- 5P. Schnider, G. Koch, R. Prétôt, G. Wang, F. M. Krüger, A. Pfaltz, Chem. Eur. J. 1997, 3, 887.
- 6R. H. Crabtree, Acc. Chem. Res. 1979, 12, 331.
- 7For other chiral pyridine and quinoline derived N,P ligands, see:
- 7aT. Bunlaksananusorn, K. Polborn, P. Knochel, Angew. Chem. 2003, 115, 4071;
10.1002/ange.200351936 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 3941;
- 7bP. Espinet, K. Soulantica, Coord. Chem. Rev. 1999, 293, 499;
- 7cJ. M. Brown, D. I. Hulmes, P. J. Guiry, Tetrahedron 1994, 50, 4493;
- 7dB. Wolfe, T. Livinghouse, J. Am. Chem. Soc. 1998, 120, 5116;
- 7eK. Ito, R. Kashiwagi, K. Iwasaki, T. Katsuki, Synlett 1999, 10, 1563;
- 7fG. Franciò, F. Faraone, W. Leitner, Angew. Chem. 2000, 112, 1486;
10.1002/(SICI)1521-3757(20000417)112:8<1486::AID-ANGE1486>3.0.CO;2-B Google ScholarAngew. Chem. Int. Ed. 2000, 39, 1428;10.1002/(SICI)1521-3773(20000417)39:8<1428::AID-ANIE1428>3.0.CO;2-K PubMed Web of Science® Google Scholar
- 7gA. V. Malkov, M. Bella, I. G. Stará, P. Koèovský, Tetrahedron Lett. 2001, 42, 3045.
- 8C. Moberg, H. Adolfsson, K. Wärnmark, P. O. Norrby, K. M. Marstokk, H. Møllendal, Chem. Eur. J. 1996, 2, 516.
- 9See, for example,
- 9aT. Imamoto, T. Kusumoto, N. Suzuki, K. Sato, J. Am. Chem. Soc. 1985, 107, 5301;
- 9bT. Imamoto, T. Oshiki, T. Onozawa, T. Kusumoto, K. Sato, J. Am. Chem. Soc. 1990, 112, 5244.
- 10H. C. Brown, J. Chandrasekharan, P. V. Ramachandran, J. Am. Chem. Soc. 1988, 110, 1539.
- 11
- 11aR. Ewalds, E. B. Eggeling, A. C. Hewat, P. C. J. Kamer, P. W. N. M. van Leeuwen, D. Vogt, Chem. Eur. J. 2000, 6, 1496 .
10.1002/(SICI)1521-3765(20000417)6:8<1496::AID-CHEM1496>3.0.CO;2-8 CAS PubMed Web of Science® Google Scholar
- 12H. C. Kolb, M. S. VanNieuwenhze, K. B. Sharpless, Chem. Rev. 1994, 94, 2483.
- 132-Vinylquinoline is commercially available from Acros Chemical, or alternately, can be prepared from quinoline in one step, see: M. A. Fakhfakh, X. Franck, A. Fournet, R. Hocquemiller, B. Figadère, Tetrahedron Lett. 2001, 42, 3847.
- 14Dihydroxylation of 2-vinylpyridine afforded the corresponding diol in only 15 % yield (94 % ee).
- 15N. Zimmermann, Dissertation, University of Basel, 2001.
- 16D. L. Reger, T. D. Wright, C. A. Little, J. J. S. Lambda, M. D. Smith, Inorg. Chem. 2001, 40, 3810. See also: J. L. Leaser, Jr.,R. Cvetovich, F.-R. Tsay, U. Dolling, T. Vickery, D. Bachert, J. Org. Chem. 2003, 68, 3695.
- 17For long-term storage (>2 weeks), complexes were kept under argon at −25 °C.
- 18CCDC-217683 (19 a), CCDC-218218 (rac-19 b), CCDC-218219 (rac-19 c) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]). rac-19 c: crystallized from CH2Cl2/Et2O, C47H50F6Ir1N1O1P2Si1, Mr=1041.16, orange block 0.20×0.22×0.24 mm, triclinic, space group P
 11, a=10.9930(8), b=12.8619(7), c=15.8814(12) Å, α=98.936(9), β=104.955(6), γ=95.555(7)°, V=2120.9(3) Å3, ρcalcd=1.630 g cm−3, μ=3.316 mm−1, F(000)=1044.000, Z=2, MoKα radiation (λ=0.71073 Å, graphite monochromator), T=173 K, Θ scan range 3.168<Θ<33.000°. 108 123 reflections collected (±h, ±k, ±l), 15 971 independent reflections, 12 910 observed reflections [I>3σ(I)], 532 refined parameters. R=0.0345, Rw=0.0242, max. residual electron density −2.55(3.06) e− Å−3, located near the metal center. There were no restraints applied. Hydrogen atoms were calculated and refined as riding atoms. Data collection was performed with a Kappa CCD diffractometer. Programs used: structure solution: SIR79 (A. Altomare, M. C. Burla, M. Camalli, G. L. Cascarano, C. Giacovazzo, A. Guagliardi, A. Grazia, G. Moliterni, G. Polidori, R. Spagna, J. Appl. Crystallogr. 1999, 32, 115), structure refinement: CRYSTALS (
D. J. Watkin, Crystals, Issue 11, Chemical Crystallography Laboratory, Oxford, 2001), weighting scheme: Chebychev polynomial (
J. R. Carruthers, D. J. Watkin, Acta Crystallogr. Sect. A 1979, 35, 698). We thank Markus Neuburger for assistance in solving the crystal structures of rac-19 b and rac-19 c.
11, a=10.9930(8), b=12.8619(7), c=15.8814(12) Å, α=98.936(9), β=104.955(6), γ=95.555(7)°, V=2120.9(3) Å3, ρcalcd=1.630 g cm−3, μ=3.316 mm−1, F(000)=1044.000, Z=2, MoKα radiation (λ=0.71073 Å, graphite monochromator), T=173 K, Θ scan range 3.168<Θ<33.000°. 108 123 reflections collected (±h, ±k, ±l), 15 971 independent reflections, 12 910 observed reflections [I>3σ(I)], 532 refined parameters. R=0.0345, Rw=0.0242, max. residual electron density −2.55(3.06) e− Å−3, located near the metal center. There were no restraints applied. Hydrogen atoms were calculated and refined as riding atoms. Data collection was performed with a Kappa CCD diffractometer. Programs used: structure solution: SIR79 (A. Altomare, M. C. Burla, M. Camalli, G. L. Cascarano, C. Giacovazzo, A. Guagliardi, A. Grazia, G. Moliterni, G. Polidori, R. Spagna, J. Appl. Crystallogr. 1999, 32, 115), structure refinement: CRYSTALS (
D. J. Watkin, Crystals, Issue 11, Chemical Crystallography Laboratory, Oxford, 2001), weighting scheme: Chebychev polynomial (
J. R. Carruthers, D. J. Watkin, Acta Crystallogr. Sect. A 1979, 35, 698). We thank Markus Neuburger for assistance in solving the crystal structures of rac-19 b and rac-19 c.
- 19While we have chosen to separate the enantiomers on expediency grounds, this is by no means necessary as enantioselective routes to all but one are known. 20 a:
- 19aB. Lipshutz, L. Asher, K. Noson, Org. Lett. 2002, 4, 4045;
- 19bR. Seemayer, M. P. Schneider, Tetrahedron: Asymmetry 1992, 3, 827;
- 19cM. D. Garret, R. Scott, G. N. Sheldrake, Tetrahedron: Asymmetry 2002, 13, 2201; 20 b:
- 19dA. G. Davies, J. Kenyon, K. Thaker, J. Chem. Soc. 1956, 3394;
- 19eN. A. Salvi, S. Chattopadhyay, Tetrahedron 2001, 57, 2833; 20 c:
- 19fC. Bolm, M. Zehnder, D. Bur, Angew. Chem. 1990, 102, 206; Angew. Chem. Int. Ed. Engl. 1990, 29, 205;
- 19gC. Bolm, M. Ewald, M. Felder, G. Schlingloff, Chem. Ber. 1992, 125, 1169;
- 19g2G. Cheulucci, F. Soccolini, Tetrahedron: Asymmetry 1992, 3, 1235.
- 20E. E. Nifantiev, M. K. Grachev, S. Y. Burmistrov, Chem. Rev. 2000, 100, 3755.
- 21We were unable to prepare the pyridyl phosphinite (R1=trityl, R2=o-Tol) even in the presence of a large excess of reagents.
- 22
- 22aO. Loiseleur, P. Meier, A. Pfaltz, Angew. Chem. 1996, 108, 218;
10.1002/ange.19961080223 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 20;
- 22bO. Loiseleur, M. Hayashi, N. Schmees, A. Pfaltz, Synthesis 1997, 1339;
- 22cO. Loiseleur, M. Hayashi, M. Keenan, N. Schmees, A. Pfaltz, J. Organomet. Chem. 1999, 576, 16.