Anion Transport by Bambusuril-Bile Acid Conjugates: Drastic Effect of the Cholesterol Content
Abstract
Artificial anion transporters offer a potential way to treat deficiencies in cellular anion transport of genetic origins. In contrast to the large variety of mobile anion carriers and self-assembled anion channels reported, unimolecular anion channels are less investigated. Herein, we present a unique example of a unimolecular anion channel based on a bambusuril (BU) macrocycle, a well-established anion receptor. The BU structure was expanded by appending various bile acid residues allowing a single molecule to span the membrane. Chloride transport mediated by BUs through lipid bilayers was investigated in liposomes and these studies revealed a surprisingly high dependence of the anion transport activity on the cholesterol content in the liposomal membrane.
The transport of anions across biological membranes is a vital process for maintaining cellular viability. This process is facilitated by anion channels and carriers;1-3 therefore dysregulation of anion transport processes can have severe consequences in biological systems.4-7 Artificial anion transporters have the potential to address deficiencies in ion transport and restore normal physiological functions by providing an alternative pathway for anion transport.8-10 In this context, bambusurils were demonstrated to be highly potent anion transporters,11 while bile acids serve as structural motifs in the construction of artificial anion transporters.
Bile acids are biomolecules synthesized in the metabolism of all vertebrates. They are characterized by distinct hydrophilic and hydrophobic faces.12 Due to their facial amphiphilicity and structural rigidity, bile acids and their derivatives are frequently employed in constructing synthetic ion channels or carriers.13-16 Well-preorganized and highly potent cholapod anion receptors and carriers were obtained when bile acids were used as a scaffold for attaching anion binding motifs such as ureas and amides.17-19 Alternatively, in “molecular umbrellas” with two or more amphiphilic bile acid-derivatives connected to a central unit, the alcohol groups of the bile acids interact with polar or anionic species, ferrying these across the membrane.20-26 Bile acids were also utilized as anchors to which a crown ether was attached via a flexible spacer, forming anchored cation carriers.27, 28 Other examples showed rod-like dimers of bile acids, of which the facial amphiphilicity promotes intramembrane self-assembly into tubular pores that span the membrane and conduct cations and anions.29-33 Furthermore, resorcin[4]arenes,34 calix[4]arenes,35-37 and other macrocycles15 have been functionalized with bile acids and their derivatives to create tubular structures to be inserted in the membrane and transport ions. However, none of these examples presents a unimolecular anion channel.
Bambus[6]urils (BUs) are a family of macrocyclic compounds capable of a high anion binding in various solvents.38, 39 Furthermore, they exhibit an efficient capability to transport anions through artificial membranes.11, 40-42 Notably, BUs featuring fluorinated benzyl groups act as efficient Cl−/HCO3− transporters, even at very low concentrations.43 However, in all the reported cases, BUs transport anions as mobile carriers moving freely inside the lipid bilayer. This is due to their relatively small height (<2 nm), which precludes the formation of a membrane-spanning channel.11 Some unimolecular anion channels have been reported,44-46 but examples based on an anion binding macrocycle are rare.47-49
Here we present the first BU-based anion channel, that has been obtained by extending the BU core with amphiphilic substituents based on bile acids. We have varied the number of hydroxy groups on the bile acid substituents to study their impact on the anion transport activity. Additionally, two different linkers between the BU and the bile acids were used to study the impact of the size of the extended compounds. Anion transport studies of these compounds revealed channel formation, as well as an unusually strong impact of the cholesterol content in the membrane on the transport activity.
To design BU derivatives which could possess anion channel properties, we decided to prolong the structure of the BU macrocycle to exceed 4 nm, to span the membrane of liposomes (Figure 1). Three different bile acid substituents including cholic acid, chenodeoxycholic acid and lithocholic acid were attached to the nitrogen atoms of the BU portals. These bile acids differ in the number and position of the hydroxy groups (Scheme 1), which influences the number of potential H-bond donors that could assist the anion transport. Despite the extensive work done on transporters based on bile acid derivatives, there is no systematic comparison of these three bile acids. We chose to attach six bile acids per BU, with three on either side, to provide an amphiphilic pathway to the BU cavity while avoiding too much steric crowding. The bile acids were attached via linkers of two different lengths, providing bile acid functionalized BUs with theoretical lengths ranging from 4.5 nm (S1, S2 and S3) up to 5.5 nm (L1, L2 and L3) to span the lipid bilayer.
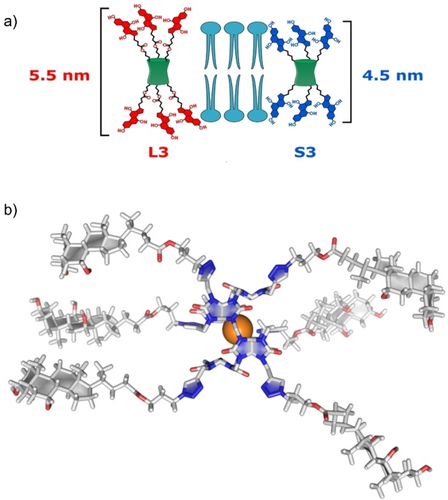
a) Schematic representation of compounds with different linkers; b) 3D model of L3 as complex with Br−, which has a calculated length of 5.5 nm.
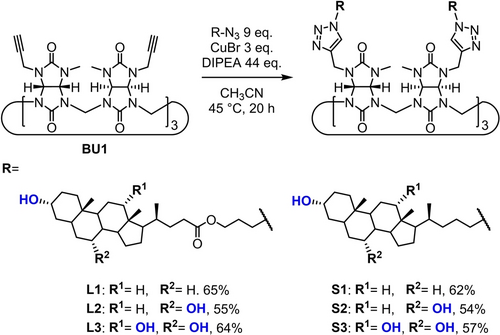
Cycloaddition reactions of propargyl BU (BU1) and azido-functionalized bile acids resulting in the target BUs. BUs connected to the bile acid residues via a longer linker are L1–L3, while the ones with a shorter linker are S1–S3. BUs conjugated with lithocholic acid are L1 and S1; with chenodeoxycholic acid L2 and S2; with cholic acid L3 and S3.
In previous work, CuAAC (Cu(I)-catalyzed azide–alkyne cycloaddition) click chemistry was used to prepare twelve-fold substituted BU macrocycles.50, 51 In this work, BU1 bearing six propargyl groups was prepared by our recently developed methodology,52 and directly conjugated to azide-functionalized bile acids by cycloaddition reactions (Scheme 1). After screening different reaction conditions (Table S1), the highest yields were obtained when CuBr was used as a catalyst and N,N-diisopropylethylamine (DIPEA) as an additive. Under these conditions, the products precipitated during the reaction and were isolated in their pure form after washing with water and acetonitrile. The desired products, BU-bile acid conjugates, were obtained in yields ranging from 54 to 65 % as their bromide complexes (Scheme 1).
The ability of these six novel BU derivatives to transport chloride was investigated in large unilamellar vesicles (LUVs). To monitor the influx of chloride anions inside the LUVs mediated by BUs, we have encapsulated the chloride sensitive probe N,N′-bis(3-sulphonatopropyl)-9,9′-biacridinium (SPBA, a lucigenin analogue) in the vesicles.53 LUVs of ~180 nm diameter (Figure S71) were prepared from 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) with the BUs preincorporated in the membranes at a concentration of 0.2 mol % with respect to the lipid concentration. LUVs with encapsulated SPBA were dispersed in a NaHCO3 solution (225 mM interior and exterior) and the experiment was started by adding a NaCl solution (25 mM) to the exterior to create a chloride gradient. We observed a quenching of the fluorescence of SPBA over time, due to the transport of chloride anions into the LUVs (Figure 2).
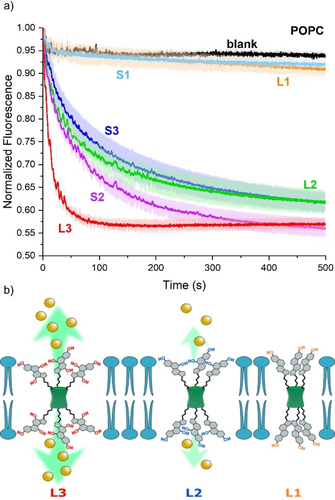
a) Chloride transport by BUs (pre-incorporated at 0.2 mol% transporter to lipid ratio) monitored by the SPBA assay in 225 mM NaHCO3, upon addition of 25 mM NaCl to the LUVs (0.4 mM lipids) made of POPC; b) Schematic representation of the compounds with different numbers of hydroxy groups, leading to different transport activities.
The transport experiments revealed a strong correlation between the transport capability of the BUs and the polarity of the bile acid substituents (Figure 2). L1 and S1 having only one hydroxy group in the bile acid substituent were incapable of any transport (orange and light blue curves in Figure 2). Increasing the number of hydroxy groups to two (L2 and S2) enhanced the anion transport significantly (green and purple curves in Figure 2). A further increase to three hydroxy groups per appended bile acid did not lead to increased activity when the shorter linker was used (S3, blue curve in Figure 2). However, with a longer linker, the most active anion transporter of the series was obtained (L3, red curve in Figure 2).
The higher transport activity with increasing number of hydroxy groups can be rationalized as follows. The conformation in which the apolar β-face of the bile acid substituents faces the lipid chains is likely to be more favorable than that in which the hydroxy group-containing α-face faces outwards. This would cause a conformation in which all three bile acid substituents on both sides of the BU would be orientated toward each other with their α-faces. As a result, a hydrophilic pore is created, enabling the conductivity of anions if at least two hydroxy groups per bile acid are present.31, 32, 54 Furthermore, more hydroxy groups should increase the hydration of the channel by interaction with water molecules, decreasing the hydrophobicity of the path which anions would need to traverse.
A longer linker connecting the triazole ring and the bile acid residue could enable the BU to easier span the membrane to form an effective channel, which can explain the higher transport activity of L3 versus S3. Additionally, the presence of an ester group in the longer linker could enhance the hydration and further contribute to the improved anion transport. On the other hand, L2 and S2 show similar transport activity regardless of the linker. The absence of the third hydroxy group on the deoxycholic derivatives might decrease the pore hydration (and increase hydrophobicity) in a part of the anion pathway, thus increasing the energetic barrier for the anion to pass the membrane. This barrier would be very similar for both L2 and S2, which would result in similar transport rates.
To confirm whether L3 and S3 function as channels or if they transport chloride as mobile carriers, we have monitored the chloride transport in LUVs prepared from 1,2-dipalmitoyl-sn-glycerophosphatidylcholine (DPPC) with BUs pre-incorporated in the membranes at 0.2 mol % using the SPBA assay. DPPC membranes show a phase transition from the solid ordered phase to the liquid disordered phase at 41 °C. If BUs act as membrane-spanning channels, the transport process should function in the solid ordered phase at temperatures below 41 °C. However, if BUs diffuse within the membrane to perform transport as mobile carriers, the transport would be stopped at low temperature with the membrane in the solid ordered phase.
For compound L3, clear transport of chloride was observed at 25 °C, which was not the case for S3 (Figure 3a). This result suggests that S3 is not long enough to span the full membrane to form a channel, while the longer L3 can function as a channel in the membrane. Planar lipid bilayer (PLB) experiments on L3 in DOPC showed some channel-like currents (Figure 3b and Figures S77–78), confirming the ability of L3 to span the membrane.
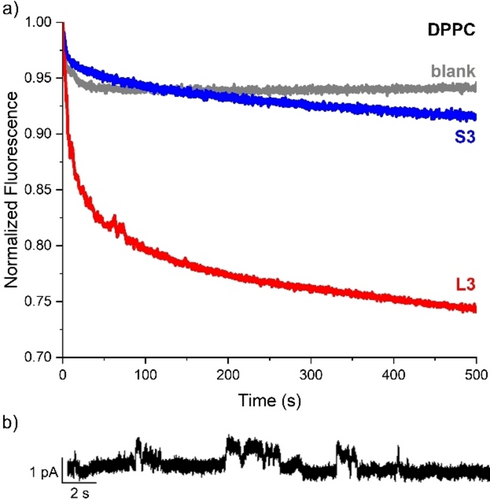
a) Chloride transport by L3 and S3 (pre-incorporated at 0.2 mol% transporter:lipid ratio) monitored by the SPBA assay in 225 mM NaHCO3, upon addition of 25 mM NaCl to the LUVs (0.4 mM lipids) made of DPPC at 25 °C; b) PLB recordings obtained for L3 in DOPC membranes at +80 mV, showing channel-like currents of ~1 pA.
Further experiments were performed on the DPPC LUVs with L3 or S3 at temperatures ranging from 15 to 45 °C. While compound S3 showed hardly any transport up to 30 °C, significant anion transport was observed at 45 °C, when DPPC is in the liquid disordered phase, allowing the free diffusion of the compound (Figure S74). This indicates that S3 transports primarily via a mobile carrier mechanism. The transport by compound L3 also increases with increasing temperature (Figure S75), indicating that mobility in the membrane enhances its activity. The compound might need this mobility for the cholic acid groups to adapt the conformation required for anion transport, which would also explain the observed variation in the channel current in the PLB experiments. The contribution of a carrier mechanism cannot be excluded either. However, the presence of clear transport activity at 25 and 30 °C, where DPPC is still in the solid ordered phase, indicates that L3 can act as a channel. We note that none of the mobile carriers tested in the lucigenin assay showed any transport activity at 25 °C,55-58 including structurally related biotin[6]uril esters and fluorinated BUs,11, 59 while at 45 °C transport was retrieved in all cases.
Inspired by an example from the literature where cholesterol was used to aid the self-assembly of the channels from molecules containing bile acids,33 we have introduced various cholesterol contents into the membranes of POPC LUVs. We repeated anion transport experiments in the SPBA assay, while gradually increasing the cholesterol to POPC ratio from 0 to 30 mol%. We observed that the introduction of cholesterol into the POPC membrane caused a dramatic decrease in the transport rates of all active BUs (Figures 4 and S73). At 30 mol% cholesterol, L3 had a 9-fold lower transport rate constant compared to POPC without cholesterol, while S3, L2, and S2 lost their transport activity completely.
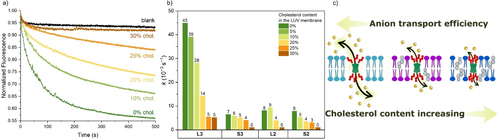
a) Chloride transport by S2 (pre-incorporated at 1:500 transporter to lipid ratio) monitored by the SPBA assay in 225 mM NaHCO3, upon addition of 25 mM NaCl to the LUVs (0.4 mM lipids) at various mol% of cholesterol in the POPC membrane of the LUVs; b) Chloride transport rate constants (k) obtained from fitting transport data of L3, S3, L2, and S2 at various cholesterol contents with a single exponential function; c) Schematic representation of the impact of cholesterol on BU-bile acid in the membrane.
This effect is much larger than usually observed in the literature (Table S3). Most commonly, a small (up to 3-fold) decrease60 in activity has been reported when the cholesterol content in the membrane was increased from 0 to 30 %28, 40, 58, 59, 61-81 Some compounds showed a larger decrease of 4–6 fold,25, 82-85 of which refences 25 and 84 also involve cholic acid derivatives. Examples of ~10-fold decrease in transport activity are rare.86, 87 We note that not only mobile carriers show decreasing rates of transport upon increasing the cholesterol content in the membrane, but this has also been observed for self-assembled anion channels (2- or 9-fold decrease at higher cholesterol content).88, 89 In contrast to the aforementioned works where at 30 % cholesterol in the membrane activity was still observed, compounds L2, S2 and S3, do not show any transport activity at this high cholesterol content. Examples where transport was absent in the presence of 30 % cholesterol for compounds showing good transport in pure POPC (almost reaching equilibration, as S3, L2, and S2 in Figure 2a) are limited to two thiosquaramides90 and three thiourea carriers,91 while other compounds in those series were less impacted.
It is well documented that increasing the cholesterol content expands the ordering of the acyl chains and decreases membrane permeability.92, 93 This increased order in the membrane could cause the bile acid substituents on the BUs to be “squeezed” together, closing the pore opening of the channel (Figure 4c). Moreover, the presence of cholesterol decreases the water content inside the membrane, especially in the closest surroundings of cholesterol molecules.94 If the bile acid derivatives would preferentially interact with cholesterol in the membrane, lower access of water molecules to the compounds could decrease the anion conductivity.
Finally, we have repeated the chloride transport experiments by L3 using SPBA in different solutions (NaHCO3, NaNO3, Na2SO4, and water without salt) to study if transport takes place via Cl−/anion− antiport or via Na+/Cl− symport (Figure S76). Good transport curves were obtained in all cases, including experiments conducted in SO42− and pure water, where antiport is expected to be difficult or impossible. This suggest that Na+/Cl− symport is the main transport mechanism.
In conclusion, we have successfully synthesized a series of six novel BUs with bile acid substituents of different polarities attached to the macrocycle via linkers of different lengths. This series of compounds provides a unique and comprehensive comparative analysis of the effect of different bile acids on anion transport when attached to the same anion-binding structure. In particular, a clear trend in transport activity based on the polarity of the bile acids was observed. More polar bile acid substituents and longer spacer groups yielded the most active transporter L3. Furthermore, L3 represents one of the rare examples of unimolecular anion channels. In addition, we found that increasing the cholesterol content strongly decreased the anion transport activities.
Our results highlight the sensitivity of transport activity to membrane composition when considering larger and more complex artificial anion transporters. The exact effect of decreased activity with higher cholesterol content in the membrane, although already observed for other transporters, has yet to be understood at the molecular level. We believe that further transport studies with higher variations in lipid composition will reveal more interesting dependencies. This would help to better understand how artificial anion transporters will behave in biological applications, as the membrane composition of cells is much more diverse than that of membrane model systems. Furthermore, the observed dependence of transport rates on cholesterol content could be further exploited to target the transport activity to cell types or organisms with a specific membrane cholesterol content.
Supporting Information
The authors have cited additional references within the Supporting Information.95-108
Acknowledgments
This work was supported by the Czech Science Foundation (23-05271S). We thank the RECETOX Research Infrastructure (LM2023069) financed by the Ministry of Education, Youth and Sports. This project was supported by the European Union′s Horizon 2020 Research and Innovation Programme under grant agreement No. 857560. MC is a Research Fellow and HV a Research Associate of the Fonds de la Recherche Scientifique—FNRS. This publication reflects only the author‘s view and the European Commission is not responsible for any use that may be made of the information it contains. We acknowledge Proteomic Core Facility of CIISB, Instruct-CZ Centre, supported by MEYS CR (LM2018127) and the National Infrastructure for Chemical Biology (CZ-OPENSCREEN, LM2023052). This article is also based upon work from COST Action CA22131, supported by COST (European Cooperation in Science and Technology). Open Access publishing facilitated by Masarykova univerzita, as part of the Wiley - CzechELib agreement.
Conflict of Interests
The authors declare no conflict of interest.




