Phase-Separated Spiropyran Coacervates as Dual-Wavelength-Switchable Reactive Oxygen Generators
Abstract
Low-molecular-weight compounds of certain structural features may form coacervates through liquid-liquid phase separation (LLPS). These coacervates can enter mammalian cells and affect cellular physiology. Controlling the properties of the coacervates inside cells, however, is a challenge. Here, we report photochemical reactions of spiropyran (SP)-based coacervates with two wavelengths of light, in vitro, in the cell, and in animals, generating reactive oxygen species (ROS) for photo-controlled cell killing. We identify an SP-containing compound, SP-PEG8-SP, that forms coacervates (SP-C) in the aqueous solution. Photo illumination by a UV light triggers the isomerization of SP to merocyanine (MC), switching SP-C to the fluorescent coacervates MC-C. A visible light converts MC-C back to SP-C and induces ROS generation. Notably, coacervate formation increases the compound's ROS generation efficiency. The SP-C/MC-C coacervate system (collectively called spiropyran coacervates) can spontaneously enter cells, and a dual-wavelength-controlled reversible on/off switch and spatiotemporal-resolved ROS production is realized within the cytoplasm. Light-induced ROS generation leads to cytotoxicity to cancer cells, tumor organoids, and tumors in vivo, supporting spiropyran coacervates’ potential use as coacervate photosensitizers in photodynamic therapies.
Introduction
Biomolecular condensates form micron-sized membraneless compartments for spatiotemporal organization of the cytoplasm to promote the activities of biochemical reactions and enrich or exclude certain molecules, thus achieving complex biological functions in cells.1-5 Increasing evidence suggests that biomolecular condensates are assembled via liquid-liquid phase separation (LLPS) driven by multivalent macromolecular interactions.1, 6, 7 Coacervate microdroplets, which are frequently employed as models for biocondensate studies, are usually assembled by biopolymers, including proteins, nucleic acids, and peptides.8-12 However, recent reports have shown that low-molecular-weight compounds such as peptides and small molecules may also phase separate to give liquid-like microdroplets in aqueous solutions.13-18 Following the principle of the “sticker-and-spacer” model, by tinkering with the interacting groups (“stickers”) at the termini and the flexibility of the linkers, one can design synthetic compounds that have a tendency to form liquid-liquid phase-separated droplets in solution.6 For example, Spruijt's group synthesized short peptide conjugates with two hydrophobic dipeptide stickers connected by a polar, flexible spacer.13, 16 These short peptide conjugates are much less complex than biopolymers, but are also able to condense into liquid droplets in the aqueous buffer and can be used to control chemical or enzymatic reactions. These work inspired us to employ the low-molecular-weight compound-based coacervates to regulate chemical reactions and control cellular activities.
Designing stimuli-responsive coacervates provides valuable tools to perturb or agitate the coacervation process, allowing the dissection of the mechanisms of molecular coacervation or the gain of biological properties. Various stimuli have been utilized, including ionic strength,19, 20 pH,21-23 temperature,24, 25 enzyme,26-29 light,17, 30-32 and bioactive molecules.33-35 However, most of these methods drastically alter the solution condition and thus irreversibly change the molecular states. For example, we designed a photoresponsive phase-separating fluorescent molecule (PPFM) based on pyrene. Light illumination cleaves a bond in the PPFM and causes irreversible coacervate dissolution.17 Light is a preferred stimulus in this example, but a reversible switch was not achieved in this photocleavage reaction. Reversible coacervate systems have been developed in vitro,36-38 but inducing coacervate transformation by light for cancer treatment in vivo has not been reported.
Molecular coacervates have been found in a variety of applications, ranging from intracellular delivery of proteins,17, 33 nucleic acids,34 or other functional molecules,26, 39 and as artificial protocells for the production or consumption of bioactive substances to regulate cellular activities.40, 41 We envision a new application of molecular coacervates that can realize photo-responsive generation of reactive oxygen species (ROS). Many diseases, such as cancer, diabetes, chronic inflammation, and Alzheimer's disease, are associated with the imbalance of ROS production in cells.42-45 Photo-induced generation of ROS in cancer cells induces cell death and has been the root of a group of emerging cancer therapies known as photodynamic therapies (PDT).46 In PDT, photosensitizers (PS) are employed to induce ROS production under the illumination of certain light. However, one of the major challenges of PDT is the unwanted off-target effect or prolonged cytotoxicity after the PDT treatment.47, 48 To overcome these limitations, developing PS that can switchably induce ROS generation is an attractive method.
Herein, we aim to develop a photo-controlled coacervate system with two properties: (1) photo-induced generation of fluorescent coacervates and (2) photo-switchable ROS generation from the coacervates. To achieve this, we designed Spiropyran (SP)-based molecules according to the sticker-and-spacer model.6, 13 Spiropyran (SP) is a highly hydrophobic molecule; under UV illumination, it can isomerize to its isomer, merocyanine (MC), which is a fluorescent photosensitizer that can induce ROS generation. This process is reversible: visible light will revert MC to SP, turning off fluorescence and ROS production.49-53 We reason that the SP compound will be a perfect system to explore dual-wavelength photo-responsiveness: UV/Vis light will interconvert the coacevates from one from to another, and induce ROS production. As molecular coacervates tend to enter cells, such a reaction can increase ROS in cancer cells and elicit anticancer effects in cells and animals (Scheme 1).
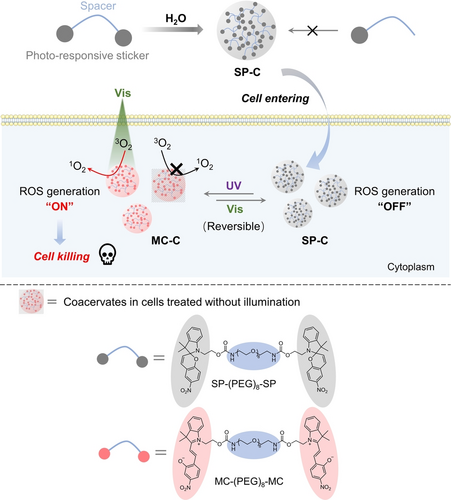
Schematic illustration showing the spiropyran (SP)-based coacervates for photo-switchable and spatiotemporally resolved ROS production and cancer cell killing. Coacervate microdroplets formed by SP-(PEG)8-SP (termed SP-C) and coacervate droplets formed by MC-(PEG)8-MC (termed MC-C) can transform between each other under UV and visible (Vis) light illuminations. Furthermore, MC-C can induce ROS production under Vis irradiation and transform back to SP-C simultaneously, whereas SP-C cannot induce ROS production. Moreover, these coacervates can enter live cells and undergo photo-induced transformation and switchable ROS production in cells, providing a potential way of treating cancers through photodynamic reactions.
Results and Discussion
Design of SP-C and the Transformation from SP-C to MC-C
According to the “sticker-and-spacer” model, small molecules consisting of two hydrophobic moieties (“stickers”) connected by a hydrophilic linker (“spacer”) tend to condense into liquid microdroplets via liquid-liquid phase separation (LLPS) under certain conditions in the aqueous solution.12, 13, 17 Here, to build photo-switchable phase-separated coacervates, we chose hydrophobic spiropyran (SP) as the sticker, as the non-fluorescent SP can undergo photo-responsive transformation to the fluorescent merocyanine (MC) form under UV light.51 Utilizing a PEG8 molecule as the linker, SP-PEG8-SP was synthesized (Figure 1a). First, we dissolve the compound SP-PEG8-SP in DMSO to form a stock solution. Then, we diluted the SP-PEG8-SP stock into water under the illumination of a 565 nm light; a white turbid solution was obtained (Figure 1b). Under a microscope, non-fluorescent microdroplets were observed (Figure 1c). Next, we stained the microdroplets with a fluorescent dye, Nile Red, and observed the fusion of small microdroplets into larger ones within 20 seconds under a fluorescent microscope (Figure S1). After we photobleached a portion of the droplet, the fluorescence intensity of the bleached area recovered within 300 seconds to 85 % of the initial fluorescence intensity before bleaching (Figures 1d, 1e). All these observations are consistent with the conclusion that the microdroplets formed by SP-PEG8-SP in aqueous solution are phase-separated coacervates (termed SP-C here) with liquid-like properties. Imaging and turbidity analysis showed that pH and NaCl concentrations affected SP-C coacervates′ size and fusion rates (Figure S2). As a control, we synthesized a PEG-modified SP monomer called SP-PEG6. We found SP-PEG6 dissolved in the aqueous solvent, showing no sign of coacervate formation (Figure S3). Taken together, we have shown a successful design of a phase-separating compound based on SP.
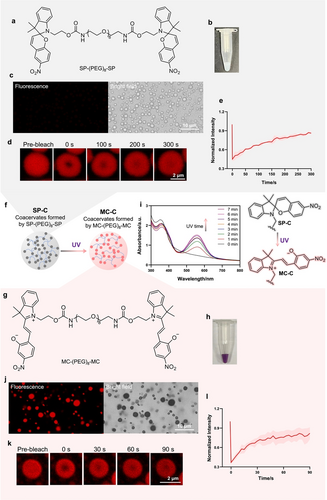
SP-based compounds and their phase-separation properties in aqueous solution. a) Chemical structure of SP-(PEG)8-SP. b) Photo, c) optical and fluorescent microscopic images of the coacervate droplets formed by SP-(PEG)8-SP. SP-C was formed in 1 : 100 DMSO/water solution under the illumination of 565 nm light (60 mW/cm2). d, e) Fluorescence recovery after photobleaching (FRAP) analysis showing the recovery of SP-C after photobleaching. SP-C was formed in 1 : 100 DMSO/water solution containing 1 mM NaCl. Note that the droplets were stained with Nile Red (5 μM). The excitation and emission wavelengths of the fluorescent images were set at 565 nm and 640 nm, respectively. f) Scheme of the transformation from SP-C to MC-C under UV (365 nm, 10 mW/cm2) light illumination. g) Structure of MC-(PEG)8-MC. h) Photo images of the coacervate droplets formed by MC-(PEG)8-MC. i) UV/Vis absorption spectrum showing the increase of absorption at 500–600 nm with the extension of UV illumination time, indicating transformation from SP-C to MC-C. j) optical and fluorescent microscopic images of the coacervate droplets formed by MC-(PEG)8-MC. k, l) FRAP analysis showing the recovery of the fluorescence of MC-C after photobleaching. MC-C was formed in 1 : 100 DMSO/water solution containing 1 mM NaCl. The excitation and emission wavelengths of the fluorescent images were set at 405 nm and 640 nm, respectively. e, l) Data are presented as the mean±s.d. of n=3 independent experiments.
Next, we examined the photo-responsive transformation of SP to MC within coacervates (Figures 1f, 1 g). Following illumination by a 365 nm light, SP-C solution turned purple, and the coacervates remained in the suspension (Figure 1h). Light illumination caused a gradual increase of a peak at 500–600 nm in the UV/Vis spectrum (Figure 1i), corresponding to the generation of MC-PEG8-MC. Also, the microdroplets turned fluorescent (Figure 1j) and could still fuse (Figure S4). In a FRAP experiment, when a part of the droplet was photobleached, the fluorescence recovered within 100 seconds (Figures 1k, 1 l). Imaging and turbidity analysis showed that pH and NaCl concentrations affected MC-C coacervates′ size and fusion rates (Figure S5). All the data above indicate that microdroplets formed by MC-PEG8-MC (termed MC-C here) are also phase-separated coacervates with liquid-like properties. Thereby, SP-C can transform into MC-C under UV light illumination.
Photochemical Reactions in Coacervates: ROS Generation and MC-C/SP-C Interconversion
Next, we tested whether photo-illumination of MC-C can induce reactive oxygen species (ROS) production under visible light. 1,3-Diphenylisobenzofuran (DPBF) was used as a probe for singlet oxygen detection; singlet oxygen will convert DPBF into 1,2-Dibenzoylbenzene (DBZB) (Figure 2a). By irradiating the mixture of DPBF and MC-C with a 565 nm light, we observed a decrease in DPBF and an increase in DBZB within 1 minute (Figure 2b). The structure of the product DBZB was confirmed using NMR (Figure S24) and mass spectrum. In contrast, irradiating the mixture of DPBF and SP-C with 565 nm light didn't cause an obvious decrease in DPBF and an increase in DBZB (Figure 2c). These results demonstrate that MC-C can induce ROS production under visible light irradiation, whereas SP-C cannot. Using DPBF as the 1O2 trapping agent and Rose Bengal (RB) as the standard compound, the 1O2 generation yield (ΦΔ) of MC-C in DI water were determined to be about 0.40 (Figure S6).

Photochemical reactions of SP-C/MC-C. a) Scheme of the photo-induced ROS production by MC-C and subsequent DPBF reaction. Briefly, a 100 mM stock solution of SP-(PEG)8-SP in DMSO was diluted in H2O under 565 nm light to give SP-C (1 mM in 1 : 100 DMSO/water); SP-C was irradiated by 365 nm light for 6 min to give MC-C. A 30 mM DMSO stock solution of DPBF was added into SP-C or MC-C to give a final concentration of 1.5 mM for photo-induced reaction. b) HPLC detection result showing the 565 nm light-induced ROS production by MC-C and subsequent DPBF oxidation in H2O. c) HPLC detection result showing no DPBF oxidation with the existence of SP-C and 565 nm irradiation in H2O. d) UV/Vis absorption spectrum showing the transformation from MC-C to SP-C under 565 nm light illumination. e) Fluorescence microscope imaging results showing the reversible transformation between SP-C and MC-C and f) the statistical average of fluorescence intensity of the droplets (data are presented as the mean ± s.d. of n=9 independent experiments). g) UV/Vis absorption spectrum showing the transformation from MC-(PEG)6 to SP-(PEG)6 under 565 nm light illumination. h) HPLC detection result showing no DPBF oxidation with the existence of MC-(PEG)6 and 565 nm light irradiation in H2O. 565 nm light: 60 mW/cm2; 365 nm light: 10 mW/cm2.
At the same time, the 565 nm light also caused the conversion of MC-C to SP-C, evidenced by the gradual decrease of the peak at 500–600 nm in the UV/Vis spectrum (Figure 2d). The transformation of SP-C and MC-C is reversible: after four cycles of switch between the two states using an alternating 365 nm and 565 nm photo illumination, only a slight loss (20 %) of the fluorescence intensity was observed (Figures 2e, 2 f).49 These data demonstrated multi-directional photochemical reactions of the coacervates: the 365 nm light turns the coacervates to MC-C, the fluorescent ROS “ON” state, whereas the 565 nm light induces ROS formation of the MC-C and at the same time shuts down the fluorescence and turns the coacervates into SP-C, the non-fluorescent ROS “OFF” state (Figure 2a).
We also observed different photochemical properties between the MC-C coacervates and the well-dispersed MC-(PEG)6 in water. Firstly, compared to MC-(PEG)6, the absorption spectrum results show a notable red shift of the MC-C absorption and a constant absorption shape lacking the appearance of a shoulder peak at around 400 nm during isomerization (Figures 2d, 2 g). Secondly, irradiating the mixture of DPBF and MC-(PEG)6 within 60 s didn't cause an obvious oxidation of DPBF (Figures 2h, S7), which suggests that the efficiency of ROS production induced by dispersed MC-(PEG)6 in water is much lower than that of the MC-C. All these results imply the J-type stacking of MC in MC-C.49, 54 However, using Rhodamine B (RhB) as the standard compound, the fluorescence quantum yield (Φf) of MC-C in DI water were determined to be 0.016 (Figure S8), which is lower than the reported values,49, 51 implying a fluorescence attenuation of the MC molecules in the coacervates. We suppose that the stacking of functional groups in coacervates can change the reaction properties in certain ways, and the coacervate state may provide a significantly different environment for the molecule compared to the soluble form.
Cell Entry and Intracellular Photochemical Reactions
We and others have shown that coacervates of small molecules or peptides can enter cells.17, 33, 39 Next, we explored whether SP-C and MC-C can enter mammalian cells and maintain their properties in cells. Fluorescence microscopy showed most of the coacervates were distributed in the cytoplasm or on the cell membrane (Figures 3a, 3b, S9), showing that both SP-C and MC-C can enter cells. It's worth noting that, after incubating SP-C or MC-C with cells in the dark for 1 day, intracellular coacervates of the two groups all showed very weak fluorescence intensity (Figures 3a, 3b), which suggests a spontaneous conversion from MC-C to SP-C inside cells at 37 °C, whereas the presence of some polar compounds in the cell may prevent the complete conversion from MC to SP.51, 55 We, therefore, call the equilibrated SP-C/MC-C coacervates spiropyran coacervates collectively. Extracellular results also proved the spontaneous transformation from MC-C to SP-C and the stability of SP-C at 37 °C (Figure S10a, S10b). However, due to the formation of the hydrogen bond, the thermal stability of MC in aqueous solution is usually higher than that of SP in aqueous solution.48, 56 For example, the UV/Vis absorption spectrum shows that water-soluble SP-(PEG)6 transforms spontaneously to MC-(PEG)6 at 37°C without illumination (Figure S10c). The opposite transformation direction of spiropyran in water solution and in coacervates is probably due to the much lower effective polarity in coacervates than in water.57 These results suggest that coacervation stabilizes SP structure in the physiological enviroment.
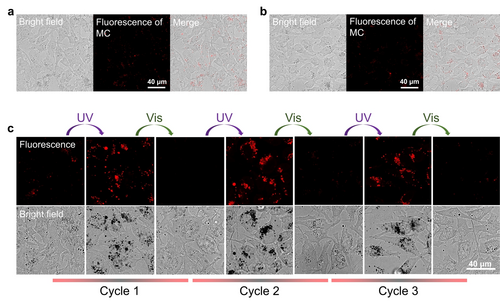
Entry of SP-C and MC-C into Hela cells and photo-induced intracellular reversible transformation between SP-C and MC-C. Briefly, Hela cells were treated with SP-C or MC-C at a concentration of 100 μM. a) Fluorescence microscope images of SP-C treated Hela cells. b) Fluorescence microscope images of MC-C treated Hela cells. c) Fluorescence microscope images of Hela cells with treatment of SP-C and illumination for different cycles, showing the reversible transformation between SP-C and MC-C in cells. UV: 365 nm, 6 min; Vis: 565 nm, 6 min.
Next, the photochemical properties of the spiropyran coacervates were detected. The fluorescence of MC-C in cells was switched on and off for at least three cycles under alternating 365 nm/565 nm light irradiation (Figure 3c), indicating that SP-C and MC-C can transform between each other reversibly under the condition of photo-illumination in cells. Then, we harnessed the power of the ROS probe, 2,7-dichlordihydro-fluorescein diacetate (DCFH-DA), to detect singlet oxygen in cells under photo illumination. The green fluorescence intensity of the oxidized product of DCFH-DA, dichlorofluorescine (DCF), is proportional to the level of ROS generated. After incubation with SP-C for 24 h, HeLa cells were incubated with DCFH-DA and then subjected to UV/Vis light illumination for different cycles (365 nm, 2 min/565 nm, 2 min) (Figure 4a). Under a fluorescent microscope, the green fluorescence from DCF was only observed with cells treated by both the coacervates and UV/Vis illumination cycles, while cells treated with only light, or only the coacervates, or coacervates and 565 nm light showed negligible DCF fluorescence (Figures 4b and S11). These results underscore the potential of MC-C to elevate intracellular ROS production under visible light, and the ability of this activation function to be turned “off” when MC-C transforms to SP-C, thus realizing an “on-and-off” switchable ROS production in cells, a significant advancement in the field. Moreover, since intracellular SP-C shows higher thermostability than MC-C (Figures 3a, 3b), undesired photosensitization of MC can be avoided.
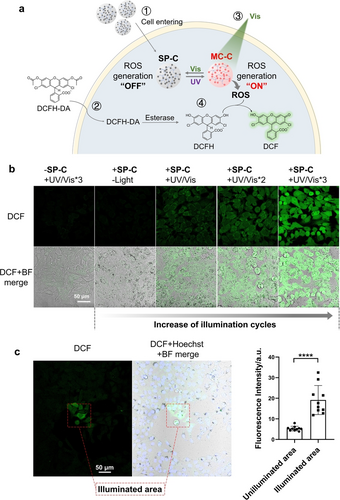
Photo-controlled spatiotemporally resolved ROS production in HeLa cells. Briefly, HeLa cells were treated with SP-C at a concentration of 100 μM and then were incubated with DCFH-DA at a concentration of 10 μM. One illumination cycle refers to irradiation under 365 nm light for 2 min and then 565 nm light for 2 min. a) Schematic illustration of the photo-induced intracellular ROS production and subsequent oxidation of DCFH: 1, entry of SP-C into cells; 2, entry of DCFH-DA into cells and esterase-catalyzed hydrolysis in the cells; 3, the transformation of SP-C to MC-C under UV light and MC-C induced ROS production under Vis light; 4, ROS induced DCFH oxidation to produce the fluorescent compound DCF. b) Fluorescence microscope images of the Hela cells treated with or without coacervates and temporal-resolved ROS production with increased UV/Vis illumination cycles. c) Fluorescence microscope images of the spatial-resolved ROS production in Hela cells treated with the coacervates and illumination, and the statistical average of fluorescence intensity of the cells in the illuminated and unilluminated area (data are presented as the mean±s.d. of n=5 independent experiments). The illuminated area in the red dotted box was treated with the laser of a fluorescence microscope, firstly by 405 nm laser for 2 min and then by 565 nm laser for 2 min.
Furthermore, we explored the spatiotemporal control of the photochemical reactions of coacervates in cells. Using the laser of a confocal fluorescence microscope as the light source, we irradiated a confined region of the cells pre-treated with SP-C first and then DCFH-DA. The fluorescence intensity of cells in the illuminated area was approximately 4 times higher than that in the unilluminated area (Figure 4c), a testament to the precision of our approach and the spatially resolved ROS production in cells loaded with the spiropyran coacervates. Besides, the fluorescence intensity of DCF increased with more irradiation cycles (Figure 4b), indicating an improvement of ROS content, which demonstrates the versatility of our method and the temporal resolution of ROS production.
Photo-Induced Cytotoxicity to Cancer Cells
After showing the potential of the spiropyran coacervates in intracellular ROS generation, we explored the use of the spiropyran coacervates to kill cancer cells, taking advantage of the cytotoxicity of intracellular ROS.46 Firstly, SP-C or MC-C at the concentration of 100 μM did not show any cytotoxicity in the dark (Figure S12). For photo-induced cytotoxicity, HeLa cells were incubated with SP-C for 24 h, illuminated with several UV/Vis illumination cycles, and then incubated for 24 h before being assayed for cell viability (Figure 5a). The results of CCK8 viability assay (Figure 5b), cell apoptosis assay (Figures 5c, S13), and LDH release assay (Figure 5d) all showed obvious cell death only in the group of treatment with both the coacervates and UV/Vis illumination cycles, while cells treated with irradiation only, coacervates only, or coacervates and 565 nm light (Figure S14) showed negligible death. These data correlate with MC-C-induced ROS production under visible light. For Hela cells, the IC50 value of MC-C under 1 illumination cycle is around 140 μM (Figure S15). Also, the cell death rate increased with increasing irradiation cycles (Figure 5b). The cytotoxicity was further proved by the result of cell morphology change (Figure 5e). Spiropyran coacervates also entered other cell lines, such as SK-BR-3 and THP1 cells (Figures S16, S17), and elicited cytotoxicity under photo illumination (Figures 5f, 5 g). Besides, the coacervates also entered HeLa cell spheroids (Figure S18), showed photo-induced ROS production (Figure S19), and demonstrated a cell-killing effect (Figure 5h). All these results indicate that the spiropyran coacervates can be used for photo-controlled cell killing and has the potential to develop into new photodynamic therapeutics. In addition, water-soluble SP-(PEG)6 exhibited significantly higher cytotoxicity than SP-C or MC-C at concentrations above 50 μM (Figure S12), suggesting that coacervation may affect the pharmacological properties of drug molecules.
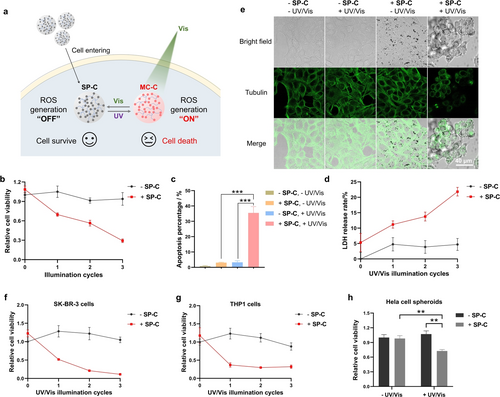
Photo-induced cytotoxicity of the spiropyran coacervates to cancer cells. Briefly, cells were treated with SP-C at a concentration of 100 μM for 24 h. The cells were illuminated for different cycles (1 illumination cycle: 365 nm, 2 min/565 nm, 2 min) and incubated for 24 h before detection. a) Schematic illustration showing photo-induced ROS generation. After entering cells, SP-C can transform into MC-C under 365 nm light illumination. MC-C then induces ROS production under 565 nm light illumination and transforms back to SP-C to turn “off” ROS production. ROS produced by MC-C can subsequently induce cell death. b) Photo-induced Hela cell killing detected by the CCK-8 assay. c) Photo-induced Hela cell apoptosis. d) Photo-induced LDH release in Hela cells. e) Photo-induced morphology change of Hela cells. f) Photo-induced SK-BR-3 cell killing detected by the CCK-8 assay. g) Photo-induced THP1 cell killing detected by the CCK-8 assay. h) Photo-induced killing of Hela cell spheroids detected by the CCK-8 assay. Cells were illuminated for three 365/565 cycles. Data are presented as the mean ± s.d. of n=3 independent experiments.
Photo-Controlled Cancer Treatment in vivo
Lastly, we explored the in vivo efficacy of coacervates for cancer treatment. The coacervates showed similar hydrodynamic sizes in the serum-containing cell cultures (around 1 μm), according to DLS and turbidity measurements (Figure S20). Then the safety of the spiropyran coacervates was evaluated. According to the routine and biochemical blood analysis, mice that received SP-C did not show changes in the levels of white blood cells (WBC), red blood cells (RBC), platelets (PLT), and hemoglobin (HGB) at either day 1 or day 15. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels remained normal (Figure S21b), indicating no toxicity to the liver. Histological examination data revealed normal cellular morphology and tissue structures (Figure S21c), suggesting that IV injection of spiropyran coacervates is well tolerated in mice.
Next, we examined the tissue distribution and tumor accumulation of the coacervates. BALB/c mice were subcutaneously implanted with 4T1 tumors. SP-C loaded with Nile Red were injected via the tail vein into tumor-bearing mice, and the mice were monitored using whole-body fluorescence imaging for 24 hours (Figure 6a). Nile Red signal appeared at the tumor site 3 hours after administration and peaked at the 6th hour before gradually diminishing (Figure 6b). Accumulation of the coacervates at the tumor site may be due to the prolonged retention of the spiropyran coacervates. Next, we dissected the tumor and performed OCT cryosectioning. Under a confocal microscope, fluorescent condensates were found in the tumor mass in the SP-C+Nile Red treatment group (Figure 6c), confirming the accumulation of the spiropyran coacervates in 4T1 tumors.
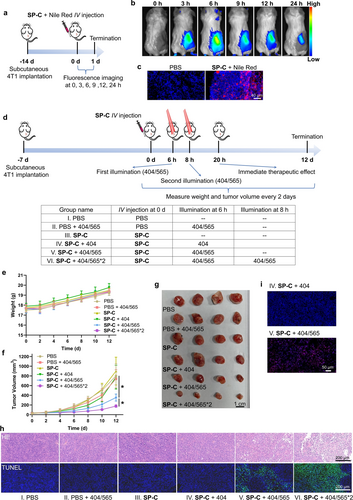
Photo-controlled tumor treatment using the spiropyran coacervates in vivo. a) Scheme showing experimental setup. Briefly, 4T1 cells were subcutaneously implanted in 5-week-old female BALB/c mice. 14 days later, 200 μL SP-C (5 mg/mL) loaded with Nile Red was administered via tail vein injection. Fluorescence imaging was performed at different time points (0, 3, 6, 9, 12 and 24 hours). b) Whole-body fluorescence imaging showing the accumulation of the spiropyran coacervates at the tumor site. c) Fluorescence images of the OCT cryosectioning of tumors showing the accumulation of the coacervates at the tumor site. d) Scheme showing the experimental setup of the six groups. 7 days after tumor inoculation, 200 μL PBS or SP-C (5 mg/mL) was administered via tail vein injection. The tumors were treated with 404 nm+565 nm, 404 nm, or no light illumination at 6 hours post-injection. For group VI, the tumors were irradiated by 404 nm+565 nm light again at 8 h post-injection. Light intensity: 0.5 W/cm2; duration: 4 min; illumination area: 35 mm2. e) Body weight measurement showing no significant toxicity of the treatments. f) Tumor volumes in different treatment groups showing the therapeutic effect of the spiropyran coacervates under UV/Vis illumination. g) Photographic images of tumors in different treatment groups 12 days after treatment. (h) HE and TUNEL staining images of the tumor dissection 20 h after SP-C injection. i) DHE staining images of the tumor cryosections 20 h after SP-C injection. Data are presented as the mean ± s.d. of n=3 independent experiments.
Next, we added a laser illumination step to induce in situ ROS generation from the coacervates at the tumor sites. Tumor-bearing mice received six different treatments: (I) PBS, (II) PBS+laser (404/565 nm), (III) SP-C, (IV) SP-C+laser (404 nm), (V) SP-C+laser (404/565 nm), and (VI) SP-C+laser (404/565*2) (Figure 6d). For group IV, we applied a laser illumination at 404 nm light to convert the spiropyran coacervates to the MC-C form. For group V, following the 404-nm light illumination, we applied a 565-nm light to elicit the ROS generation immediately. For group VI, in order to enhance the efficacy of ROS generation, we repeated 404/565 illumination at a laser power of 0.5 W/cm2 for 4 minutes 6 hours and 8 hours after the drug administration, respectively. We observed that mice in all the groups behaved normally, and the body weights increased in 12 days (Figure 6e). Tumor growth in groups V and VI showed a remarkable decrease compared to groups I–IV, with the smallest tumor sizes observed in group VI (Figures 6f and 6 g). These data indicate that photo-induced in situ ROS generation is responsible for the tumor growth suppression effect. This conclusion was further supported by TUNEL and HE staining of tumor tissues: groups V and VI, massive cell necrosis and atrophy were observed, confirming the tumor damaging effect (Figure 6h). Lastly, we performed a DHE (dihydroethidium) assay to directly measure ROS levels in live cells. A comparison of groups IV and V showed a significantly higher signal in tumors that have received 565 nm light illumination (Figure 6i), consistent with our notion that the ROS induced by 565 nm light from the MC-C form was responsible for tumor cell killing and tumor growth suppression. Notably, although the cytotoxicity of MC-C was modest in vitro (IC50 measured to be 140 μM in cell-based experiments), we observed a pronounced tumor-killing effect in vivo. We reason that the enrichment of MC-C at the tumor sites might explain such enhanced cytotoxicity in vivo. Taken together, these data prove that spiropyran coacervates have a strong tumor-site enrichment via IV injection, and a dual-wavelength switch of ROS production inhibits tumor growth and causes significant tumor cell killing.
Conclusions
In summary, we report SP-based compounds as a new class of coacervating compounds in the aqueous solution. Dual-wavelength-controlled photochemical reactions convert the coacervates between SP-C and MC-C in a reversible and controllable manner, and induce ROS generation in vitro, in cells, and in animals. The spiropyran coacervates showed exceptional chemical properties, including 1) improvement of ROS production compared to water-soluble MC molecules; 2) “on-and-off” switchable ROS production in the aqueous solution, in cells, and in animals; 3) spatiotemporal-resolved ROS production in cells and organoids; 4) effective killing of cancer cells both in vitro and in vivo. To our knowledge, this is the first example that utilizes coacervate as a ROS generator for cell killing, providing a new venue for constructing biocondensate mimics for cell manipulation and photodynamic therapy. Small-molecule coacervates show distinct chemical, physical, and pharmacological features compared to the soluble, discrete form, which may provide advantages as a new type of therapeutics.
Acknowledgments
This work was partially funded by grants from the National Natural Science Foundation of China (Grant No. 62305121), University Grants Committee of Hong Kong (GRF grants 14304921, 14306222, and 14307523), Research Impact Fund R5013-19, and CUHK (ICSG, CRIMS, and Direct Grant 4053563).
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




