Interception and Synthetic Application of Diradical and Diene Forms of Dual-Nature Azabicyclic o-Quinodimethanes Generated by 6π-Azaelectrocyclization
Abstract
We demonstrate that 2-alkenylarylaldimines and ketimines undergo thermal 6π-azaelectrocyclization to generate a wide range of azabicyclic o-quinodimethanes (o-QDMs). These o-QDMs exist as a hybrid of a diene and a benzylic diradical. The diradical nature was confirmed by their ability to undergo dimerization and react with H-atom donor, 2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) and O2. In addition, the interception of the diradicaloid o-QDMs by H-atom transfer was used to synthesize five tetrahydroisoquinoline alkaloids and related bioactive molecules. The diene form can undergo [4+2] cycloaddition reactions with different dienophiles to generate bridged azabicycles in high endo:exo selectivity. The azabicyclic o-QDMs can be generated for [4+2] cycloaddition from a wide range of electronically and sterically varied 2-alkenylarylimines, including mono, di, tri and tetrasubstituted alkenes, and imines derived from arylamine, alkylamine (1°, 2°, 3°), benzylamine, benzylsulfonamide and Boc-amine.
Introduction
o-Quinodimethane (o-QDM), a transiently dearomatized species that capitalize on susceptibility to aromatization for reactivity, is an important intermediate in organic synthesis.1 This intermediate has been studied in details for its existence and characteristics, and exploited in a number of synthesis of complex molecules, including steroids,2 alkaloids,3 and polyaromatic hydrocarbons.4 Yet, there are only a limited number of preferred routes for its generation and the intermediate lacks skeletal variation from the canonical (o or p)-xylenyl structure (Scheme 1a). The most common methods for the synthesis of the (o or p)-xylenyl forms include the high temperature (>180 °C) and flash vacuum thermolysis and high energy (UV) photolysis for breaking cyclobutane in benzocyclobutane and eliminating leaving groups, such as halogens, silanes, amines and alkyltins from α,α’-disubstituted-o-xylenes.5 Additional methods include the cyclic organic and organometallic variants of α,α’-disubstituted o-xylenes, yet, again, requiring thermolysis and photolysis for the elimination SO2, CO2, N2 and metals.6 Enolizable and trialkylstannylated (SnBu3) benzylic alkyls are also amenable for deprotonative and eliminative enolization to generate o-quinodimethanes photochemically,7 by electrolysis,8 and in the presence of strong bases and electrophiles, such as lithium diisopropylamide (LDA), Zn/TMSCl, tetrabutylammonium fluoride (TBAF) and PhSeR.9 Recently, Matsuya10 and Melchiorre11 generated o-QDMs in azidobenzocyclobutene and 2-methylindole-3-acrylaldehyde by the formation of iminophosphoranes and enamines, respectively, under mild conditions.
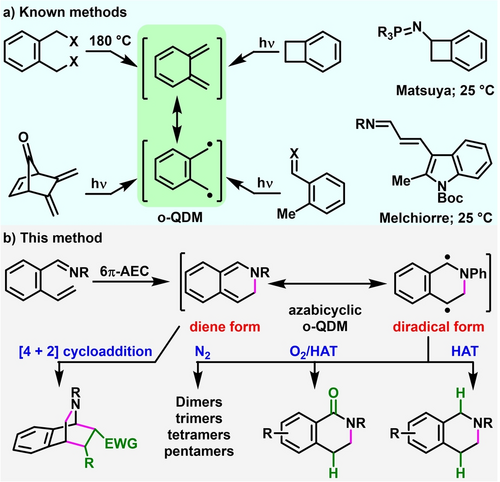
Existing methods and the current method to generate o-quinodimethane intermediates.
o-QDMs are also proposed to exhibit dual character with contributions from a diene and a benzylic diradical. The diene is the major contributor and the diradical is the minor contributor. As such, the reactivity of most o-QDMs has been consistent with the diene structure, such as in the symmetry-allowed thermal [4+2] cycloadditions. The diene forms have even been observed by spectroscopic methods including flow NMR12 and low temperatures,13 and characterized by X-ray.14 In contrast, the identification of the diradical character of the canonical o-QDM by experiments is rare15 often invoking their photolytic generation from benzocyclobutene as evidence and has been largely proposed based on absorption measurements and quantum chemical calculations.16, 1b o-QDMs embedded in extended π-systems of polyaromatics are known to demonstrate diradical nature17 and undergo symmetry-forbidden thermal [4+4] cycloaddition in limited cases.17a, 18 Except these rare observations in a specific π-extended system, the interception and demonstration of useful synthetic application of the diradical form of simple o-QDMs is not known yet.
Herein, we disclose a new route to generate conveniently azabicyclic o-QDMs through 6π-azaelectrocyclization and experimentally demonstrate its dual characteristics as an azabicyclic diene by [4+2] cycloaddition and benzylic diradical through dimerization, radical trapping with O2 and TEMPO, and H-atom transfer reactions (Scheme 1b).19 We further demonstrate the utility of the diradical form by the synthesis of five tetrahydroisoquinoline alkaloids and that of diene form by the synthesis of bridged azabicycles by [4+2] cycloaddition with diverse dienophiles, and electronically and sterically varied 2-alkenylarylimines.
Results and Discussion
During our studies on alkene difunctionalization,20 we recognized that the syn-geometric disposal of the aldimine and alkene in 2-alkenylaryladimines (1–2) was electronically poised to undergo thermal 6π-azaelectrocyclization that could potentially generate azabicyclic o-QDMs. Indeed, aldimine 1–2 underwent cyclization at 120 °C in dioxane and generated unsymmetrical and symmetrical dimers 3–6 with 6-H and 6-CF3 in 38 % (~1 : 1 ratio) and 25 % (~1 : 1.5 ratio) yields along with the detection of trimers, tetramers and pentamers by high resolution mass spectrometry (HRMS) (Scheme 2). The structures of the dimers 3–6 were characterized by X-ray crystallography (Figure 1) in addition to NMR spectroscopy. The formation of the symmetrical and unsymmetrical dimers is consistent with the diradical nature of o-QDMs. We also found that TEMPO as a radical interceptor21 and butylated hydroxytoluene (BHT) as an H-atom donor22 give complementary regioselectivity with the former preferentially generating symmetrical and the latter forming unsymmetrical dimers in good yields. In addition to the dimers 3–6, TEMPO also served as an O-donor23 and generated 1-isoquinolinones 7 and 8 in 18 % and 22 % yields, along with the detection of TEMPO adducts 10 and 11, and tetramethylpiperidine (TEMP) by LCMS and HRMS, indicating further trapping of the benzylic diradical with the radical source followed by oxidation and elimination.
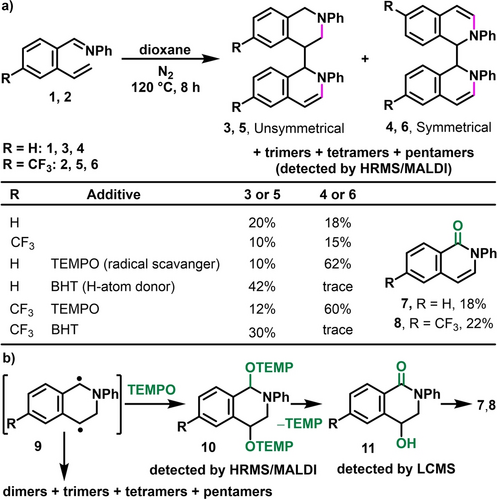
Evidence for the existence of benzylic diradicals. a) Dimerization. b) Interception by TEMPO.
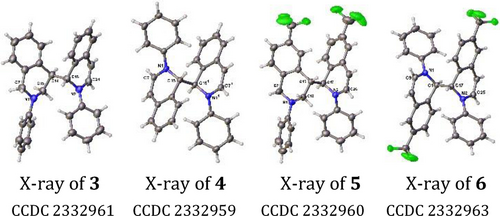
X-ray structures of unsymmetrical and symmetrical dimers 3–6.
We conducted additional experiments to garner support for the existence of benzylic diradicals (Scheme 3). We demonstrated that the benzylic diradicals could be intercepted by an H-atom and an O-radical. In the presence of triethylamine (TEA), a known H-atom donor by e-transfer/proton-transfer mechanism,24 under N2, the benzylic diradicals were intercepted to create tetrahydroisoquinolines 12–14 as products only in trace amounts and their corresponding dimers were observed (Scheme 3a). In the presence of 4-FC6H4SH as an additional H-atom donor for benzylic radicals25 along with TEA, the yields of the products 12–14 increased to 84–90 %. The use of 4-FC6H4SD generated product 12 with 56 % and 27 % deuterium at benzylic sites C1 and C4 (112 % and 54 % D incorporation), respectively, along with the dimer (4-FC6H4S)2 in 60 % yield.26 The reaction condition was also applicable to internal alkenes containing cyclopropyl (15) and cyclobutyl (16) rings as well as to a ketimine (17). Under O2, the reaction in the presence of H-atom donors (triethylamine/4-FC6H4SH) furnished 1-dihydroisoquinolinones 18–20 in 29–39 % yields through the reaction of O2 with the benzylic radical at C1 position (Scheme 3b). At reduced concentration of O2 (air), a peroxide intermediate 23 can be isolated and fully characterized by NMR and X-ray crystallography along with the products 13 and 19, further confirming the existence of the peroxy radical intermediates (such as 22) upon reaction of the benzylic radicals with O2.
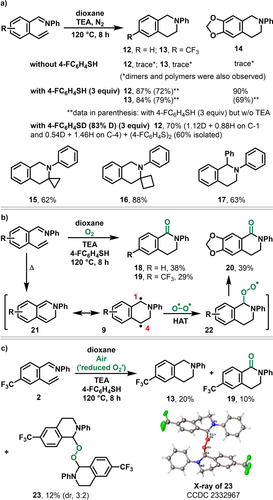
Reactions of benzylic diradicals with triethylamine/4-FC6H4SH as a H-atom donor and O2.
We further sought to exploit the ability of intercepting the diradicaloid o-QDMs by H-atom transfer (HAT) to synthesize tetrahydroisoquinoline alkaloids27 and related bioactive molecules directly from simply arylaldimines (Table 1).28 This class of alkaloid displays a wide range of biological properties, including as antineutroinflammatory29 and anticancer antibiotic activities.30 Our initial attempt to synthesize ticlopidine (Ticlid®) 24,31 a commercial medicine to prevent strokes, from an imine derived from 2-vinylthiophene-3-carbaldehyde and 2-chlorobenzylamine led to benzylic thioetherification potentially due to reaction at the benzylic position with 4-FC6H4S⋅. We were able to address this issue and synthesize ticlopidine 24 by simply switching 4-FC6H4SH to its sterically encumbered variant, 2,4,6-triisopropylthiophenol, to prevent the radical recombination with ArS⋅. Moreover, we also utilized imines derived from 2-vinyl-4,5-dimethoxybenzaldimine with 3,4-dimethoxybenzylamine, 3,4-methylenedioxybenzylamine and 4-fluorobenzylamine, respectively, to prepare intebremine 25, intebrinine 26 and MAO-B-In-15 (a MAO-B inhibitor) 27.28b, 32 Likewise, we were also able to convert an imine derived from 2-vinyl-4,5-methylenedioxyphenylcarbaldehyde with 4-methoxybenzylamine to another alkaloid viguine 28.33 After confirming the formation of azabicyclic o-QDMs and their diradical nature, we proceeded with conducting [4+2] cycloaddition reactions to intercept them in their 1,3-diene form. As such, when aldimine 1 was heated with N-phenylmaleimide at 130 °C in dioxane for 3 h, the [4+2] cycloaddition product 29 was produced in 95 % yield (Table 2, entry 1) via the formation of two carbon-carbon (C−C) bonds across the dibenzylic 1- and 4-positions of the azabicyclic o-QDM (such as 21). The reaction can be readily scaled up to gram scale without loss in yields (14.48 mmol, 5.07 g, 92 %).
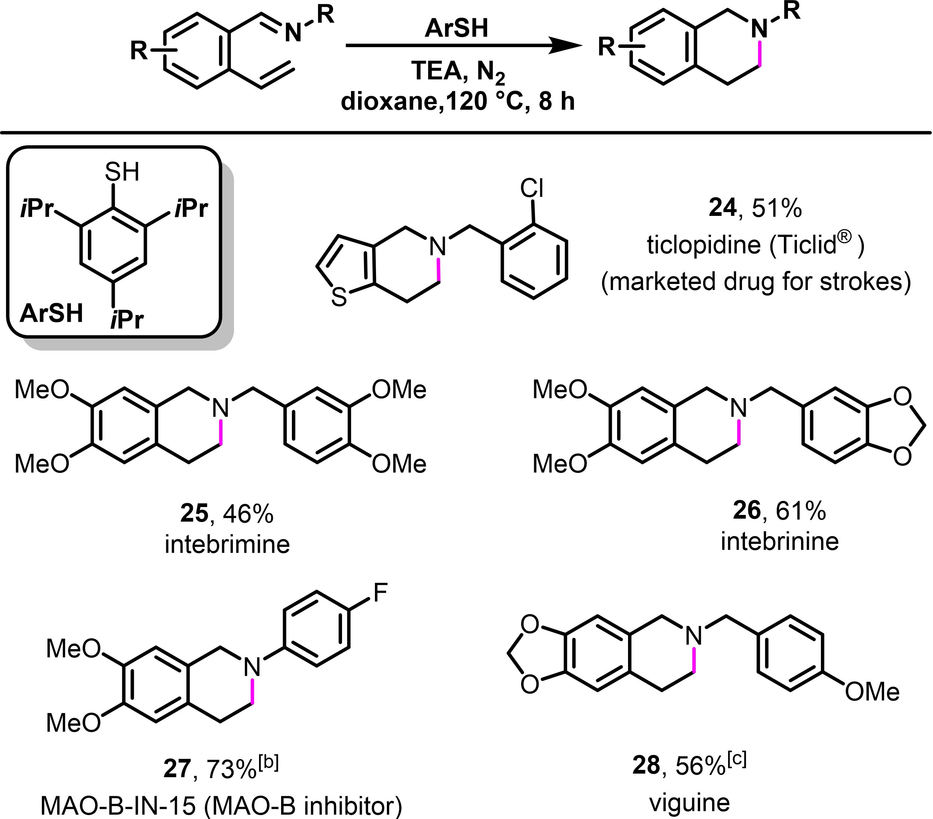
- [a] Conditions: 0.50 mmol scale reaction in 3 mL solvent with 3 equivalents of ArSH. [b] 4-FC6H4SH was used. [c] In situ generated imine was used directly without purification.
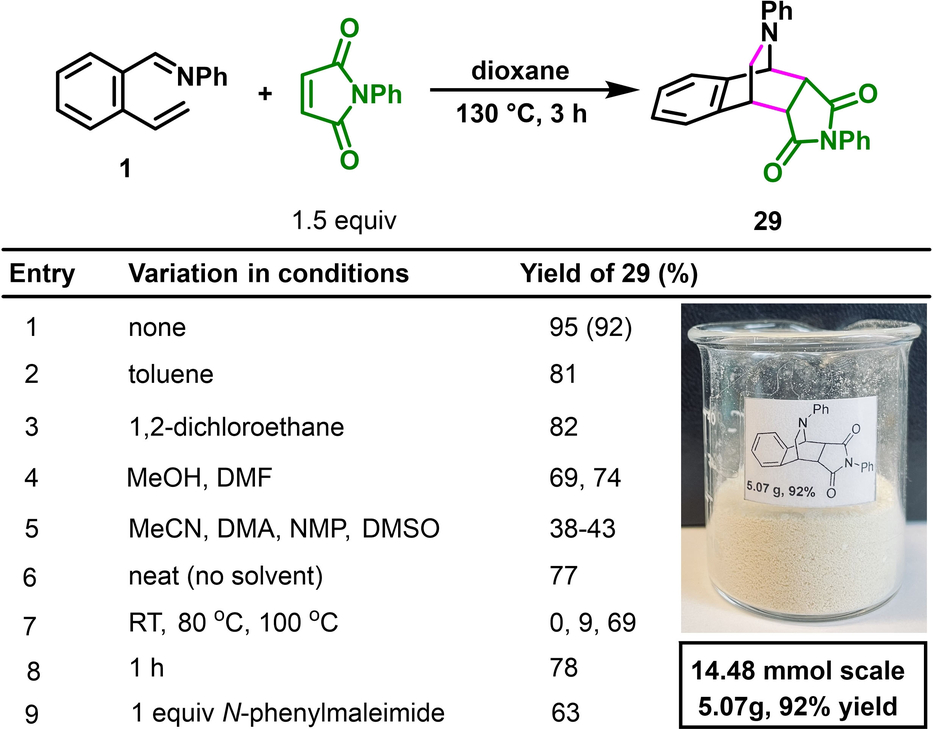
- [a] Conditions: 0.20 mmol scale reaction in 1 mL solvent. NMR yields with tetrachloroethane as a standard. Yield of the isolated product in parenthesis.
The reaction can be conducted in toluene, DCE, MeOH and DMF in similar yields (69–82 %) (entries 2–4). The reaction proceeded in moderate yields (38–43 %) in other solvents like MeCN, DMA, NMP and DMSO (entry 5). The reaction could also be run without solvent (neat), at 100 °C, in 1 h or with 1 equivalent of N-phenylphthalimide to obtain the product in good yields (entries 6–9).
After establishing the optimal reaction parameters, we examined the scope of o-QDMs for [4+2] cycloaddition (Table 3). The azacyclic o-QDMs could be intercepted with a range of cyclic dienophiles, such as maleic anhydride, maleimide, N-alkyl (methyl, t-butyl, cyclohexyl) maleimide and N-aryl maleimide (30–35) in high endo selectivity. The azacyclic o-QDMs also tolerate acidic protons as shown by the [4+2] cycloaddition with N-(4-hydroxyphenyl)maleimide (35). The o-QDMs were also reactive with acyclic symmetric dienophiles like trans-1,2-dibenzoylethylene (36). Unsymmetric dienophiles such as methyl vinyl ketone and methyl acrylate furnished the [4+2] cycloaddition products (37–38) as a mixture of endo and exo products in moderate selectivity. Next, we examined the scope with regard to different electronic and steric variations on 2-alkenylaldimines. The reaction tolerates both electron rich and deficient substituents, such as methyl, tert-butyl, methoxy, dimethoxy, 1,3-dioxanyl, fluoro, difluoro, trifluoromethyl and nitro, at different positions on the aldiminyl arenes (39–51). Likewise, the 2-alkene in 2-alkenylaldimine can also be varied with methyl, phenyl, ester and benzyl ether at the α and β-positions (52–58), demonstrating that both disubstituted terminal (52–54) and internal (55–58) alkenes can participate in the reaction. More sterically hindered tri- and tetrasubstituted 2-alkenes bearing two and three methyls, carbocycles and N,O-heterocycles at both the α- and β-positions also undergo 6π-electrocyclization followed by [4+2] cycloaddition (59–66), showcasing the robustness of this method and high reactivity of azacyclic o-QDMs to create highly hindered bridged azabicyclic structures. The reaction also shows a high level of compatibility with substituted anilines on the imine. For example, imines derived from anilines substituted at ortho, meta and para-positions with methyl, methoxy, dimethoxy, thiomethyl, chloro, bromo, iodo and meta-dinitro groups, are excellent substrates for [4+2] cycloaddition (67–74). Imines derived from polyaromatic amines (75) and heteroaryl amines (76) are also compatible for the reaction. Reactions can also be conducted with 2-alkenylketimines derived from 2-alkenylacetophenone and 2-alkenylbenzophenone (77–78).
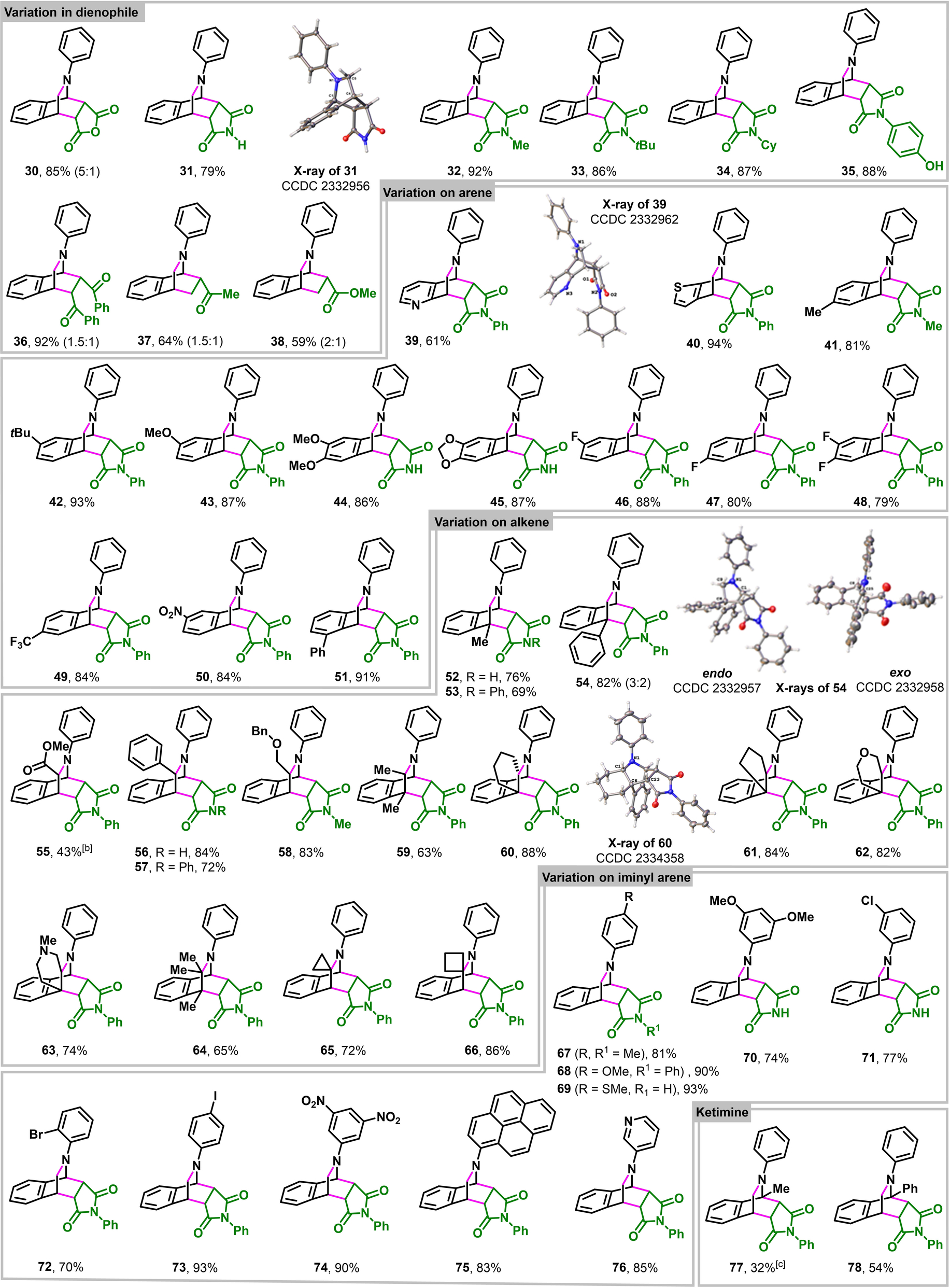
- [a] Reactions were run with 0.50 mmol imine and 0.75 mmol dienophile in 3.0 mL dioxane at 130 °C for 3 h. Unless stated otherwise, the ratio in parenthesis represents endo:exo. [b] Product 55 racemized to a 1 : 1 ratio during purification by column chromatography. [c] In situ generated imine (1.0 mmol) was used directly without purification.
The generation of azabicyclic o-QDMs is not limited to arylimines only (Table 4). Arylimines derived from benzylsulfonamide and Boc-amines also generate azabicyclic o-QDMs, which underwent [4+2] cycloaddition with N-phenylmaleimide under the standard conditions to generate corresponding bridgehead azabicycles in excellent yields (79–80). Likewise, imines derived from primary, secondary and tertiary alkylamines including benzylamine were also compatible for the reaction (81–85). Alkyl imines derived from pharmaceuticals and natural products, such as (-)-cis-myrtanylamine (86) and celacoxib (87), also furnished the bridgehead azabicycles in excellent yields.
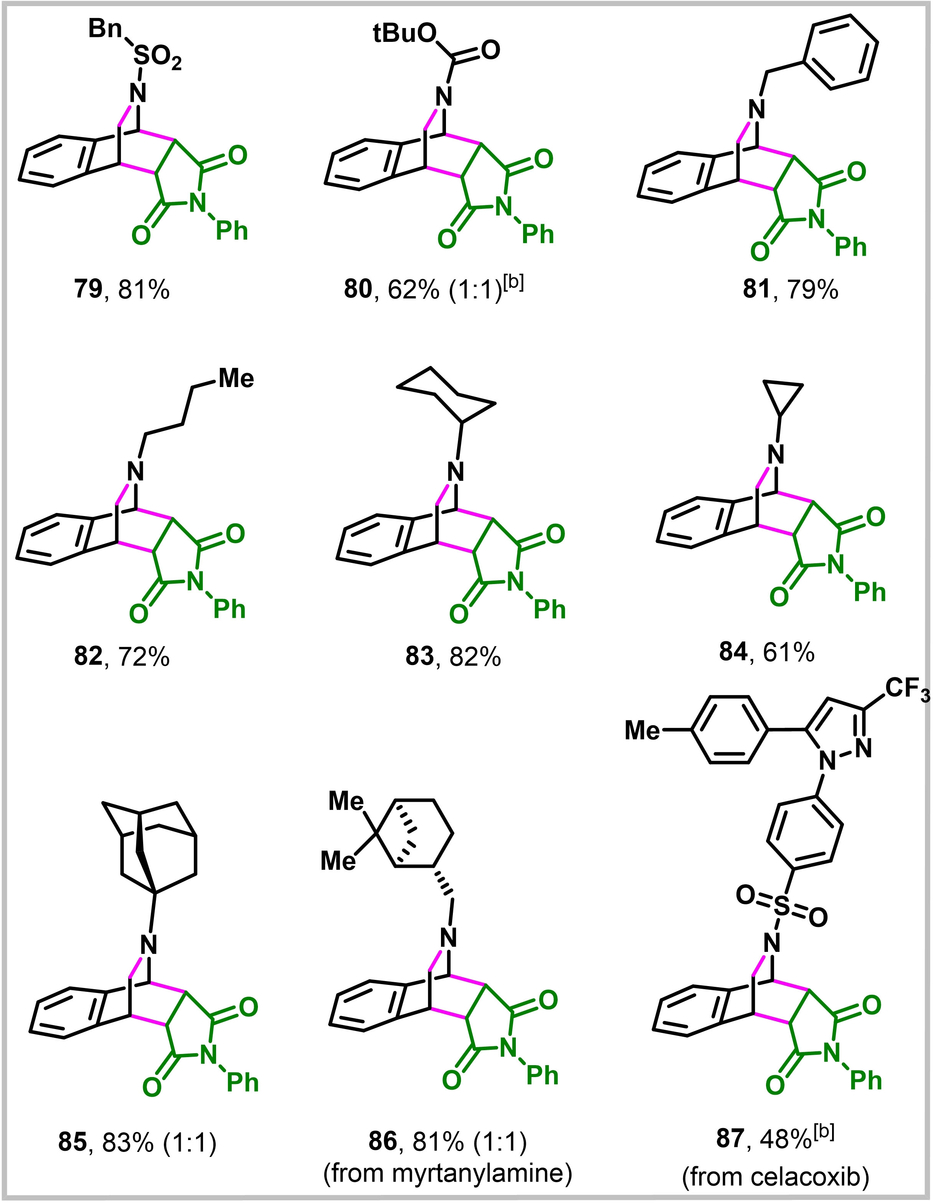
- [a] Reactions were run with 0.50 mmol imine and 0.75 mmol N-phenylmaleimide in 3.0 mL dioxane at 130 °C for 3 h. Unless stated otherwise, the ratio in parenthesis represents endo:exo. [b] In situ generated imine (1.0 mmol) was used directly without purification.
Conclusion
In summary, we have demonstrated that 2-alkenylarylaldimines and ketimines could undergo 6π-azaelectrocyclization under thermal conditions to generate azabicyclic o-QDMs. These o-QDMs show dual-nature characteristics as a cyclic diene and a benzylic diradical. We furnished strong evidence for its diradical nature by its reaction with H-atom donor, TEMPO and O2 which intercepted the carbon radicals. The interception of the diradicaloid o-QDMs by H-atom transfer also enabled us to synthesize five tetrahydroisoquinoline alkaloids and related bioactive molecules. Its characteristics as a cyclic diene was confirmed by [4+2] cycloaddition reactions that generated bridgehead azabicycles. The cycloaddition reaction showed a broad substrate scope with different dienophiles, and tolerated sterics and a range of functional groups on the arene, alkene and imine components derived from both aryl, and 1°, 2° and 3° alkylamines including those based on natural products.
Supporting Information
Deposition numbers 2332961 (for 3), 2332959( for 4), 2332960 (for 5), 2332963 (for 6), 2332967(for 23), 2332956 (for 31), 2332962 (for 39), 2332958 (for 54-exo), 2332957 (for 54-endo) and 2334358 (for 60) contain the supplementary crystallographic data for this paper. These data are provide free of charge by the Cambridge Crystallographic Data Center (CCDC).
Acknowledgments
We gratefully acknowledge the NSF (CHE-2102394), the NIH NIGMS (R35 GM133438) and The Pennsylvania State University for support of this work. The X-ray instrument was funded by the NIH SIG S10 grants (1S10OD028589-01 and 1S10RR023439-01).
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The experimental and compound characterization data for new products are available in the supplementary material of this article.




