Enantioconvergent and Site-Selective Etherification of Carbohydrate Polyols through Chiral Copper Radical Catalysis
Abstract
Going beyond currently reported two electron transformations that formed the core backdrop of asymmetric catalytic site-selective carbohydrate polyol functionalizations, we herein report a seminal demonstration of an enantioconvergent copper catalyzed site-selective etherification of minimally protected saccharides through a single-electron radical pathway. Further, this strategy paves a rare strategy, through which a carboxamide scaffold that is present in some glycomimetics of pharmacological relevance, can be selectively introduced. In light of the burgeoning interest in chiral radical catalysis, and the virtual absence of such stereocontrol broadly in carbohydrate synthesis, our strategy showcased the unknown capability of chiral radical copper catalysis as a contemporary tool to address the formidable site-selectivity challenge on a remarkable palette of naturally occurring saccharides. When reducing sugars were employed, a further dynamic kinetic resolution type glycosylation can be activated by the catalytic system to selectively generate the challenging β-O-glycosides.
Introduction
Tackling the site/regio-selectivity challenge is broadly recognized as one of the most fundamental but yet vexing challenges in synthesis.1 The relevance of this problem stems from the repetitive presentation of numerous chemically equivalent but stereochemically unique functional groups in biologically relevant molecules. By virtue of the prevalence of chiral alcohols in a plethora of biological scaffolds, seeking good synthetic solutions to precisely functionalize one hydroxyl group out of many is of utmost importance. In particular, the chiral catalytic discrimination of a carbohydrate polyol2 has thus gained substantial attention as it enables a direct and targeted C−O functionalization without requiring tedious protection/deprotection strategies that inevitably lengthen synthetic routes and generate unnecessary chemical waste.
To date, there are notable advances in catalytic site-selective carbohydrate C−O functionalizations (Figure 1A) which spans from transition metal2c to organocatalytic strategies.2a For the former, the synergistic combination2h of a chiral transition metal catalyst with an organoboron reagent had witnessed substantial strides by works from Niu3, Tang4 and our group5 in the last five years. In some rare cases reported by Niu6 and Tang7, omitting the organoboron catalyst was also feasible. For the latter, benzotetramisole catalysis,8 organoboron catalysis,9 bis-thiourea catalysis,10 and N-heterocyclic carbene catalysis11 had contributed solid solutions towards regioselective carbohydrate functionalizations and in the majority of cases, acylations.12 Despite this multitude of advances, all of these currently reported site-selective C−O bond formations on the carbohydrate polyol scaffold are mechanistically grounded on two electron ionic processes.
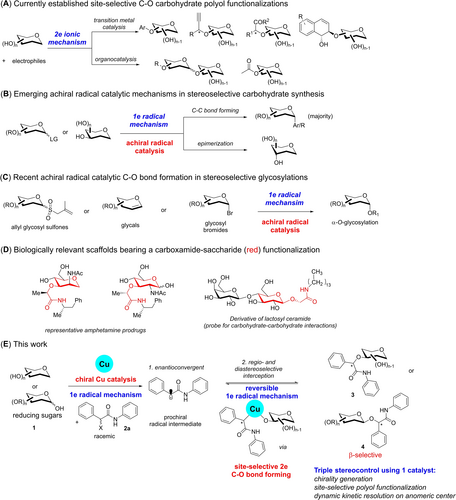
State-of-the-art in site-selective carbohydrate C−O functionalizations and emerging radical catalytic methods in carbohydrate synthesis. (A) Known site-selective carbohydrate functionalizations using organo- and transition metal catalysis through 2 electron ionic mechanisms. (B) Emerging radical reactions using achiral catalysis in C-glycosylations or epimerizations through 1 electron mechanisms. (C) Recent achiral radical catalysis in stereoselective α-O-glycosylations. (D) Biologically relevant scaffolds bearing a carboxamide-saccharide motif. (E) This work.
On the other hand, despite a surge of developments pertaining to radical functionalizations in carbohydrate synthesis (Figure 1B),13 these methods are still largely constrained to achiral radical catalysis. Asymmetric radical catalysis derived stereocontrol is still an unexplored topic broadly in carbohydrate synthesis despite its emerging importance in enantioselective transformations.14 Transformation wise, reported achiral catalytic instances encompass largely C−C bond formations on the anomeric center15 and the skeletal framework;16 as well as epimierizations to rare sugars.17 In contrast, catalytic stereoselective C−O bond formations that possess a radical nature is surprisingly scarce despite its enormous potential in accessing unprecedented pathways. Notably, stereoselective catalytic C−O transformations involving radical mechanisms (Figure 1C) were only realized lately by the Niu group using allyl sulfone donors,18 the Ye group using glycal donors19 and the Nguyen group using glycosyl bromides.20 Despite these elegant advances, these examples also involve only achiral catalysts, and most do not address the site-selectivity issue of minimally protected saccharides. Further, α-O-glycosides were generally formed by virtue of the formation of the thermodynamically favored anomeric radical. A non-catalytic exception is a base promoted β-O-glycosylation of carboxylic acids lately described by Niu.21 Importantly, an asymmetric catalytic site-selective C−O functionalization of carbohydrate polyols that harnesses a radical mechanism is still elusive.
Taking inspiration from late advancements in enantioconvergent copper catalyzed radical methods in C−O bond constructions,22 we were intrigued by the possibility of tapping into the unharnessed potential of this activation mode to surmount the multi-faceted selectivity challenges faced in site selective carbohydrate synthesis. Based on our laboratory experience and literature reported instances, chiral catalytic systems that performed well in enantioselective reactions frequently do not translate directly to the robust addressing of site-selectivity in carbohydrates due to the diverse stereochemical profiles and unique reactivity characteristics of sugars. Further, the overwhelming substrate control of sugars either through stereoelectronic or steric effects23 frequently blunts the effectiveness of the catalyst to induce stereoselectivity. Therefore, while significant pioneering developments by Liu et al.,24 Fu et al.25 and Liu et al.26 offer exciting impulses using chiral copper catalytic/bis-oxazolidine (BOX) ligand platforms in the enantioselective realm, the question as to whether the spatial asymmetry imparted by radical copper catalysis mechanisms in C−O bond formation could also be effective in concomitantly addressing the long-standing site-selectivity issue2a-2c, 2e, 27 in carbohydrate synthesis still remains unanswered.
Concurrently, the development of stereoselective methods that introduce an amide motif onto a minimally protected saccharide is surprisingly rare despite known useful applications of analogous functionalizations (Figure 1D). For example, 3-OH functionalizations of mannosyl and glucosyl scaffolds with an amide on the α-amidic carbon are documented to be useful in the development of amphetamine prodrugs which enable amphetamine release in the colon.28 Further, the β-O-anomeric functionalization with an amide motif is a scaffold present naturally in glycolipids such as lactosyl ceramide.29 Such carboxamide-saccharide derivatives were also reported to be useful probes for carbohydrate-carbohydrate interactions studies with micelles.30 Additionally, amide functionalization on saccharides had found applications in the synthesis of glycosylated asterisk ligands that inhibit Concanavalin A induced hemagglutination.31
Herein, we report the seminal exploitation of chiral radical copper catalysis in the enantioconvergent and site-selective etherification of carbohydrate polyols with racemic α-haloamides (Figure 1E). Our strategy showcases a remarkable case where a chiral catalytic platform can be harnessed to intercept a prochiral radical intermediate converged from its racemic haloamide precursor 2 a. A subsequent series of reversible elementary steps which contain single electron transfer (SET) for C-centered radical interception, is pivotal in assembling the correct stereochemical geometry prior to the asymmetric C−O bond forming step (see below, see Figure 3A for details). It is important to note that while previously reported instances involve 1e processes on the carbohydrate moiety (Figure 1B and 1 C), our catalytic radical mechanism does not involve 1e C-centered radical generation on the carbohydrate carbon framework. Instead, the single electron processes are intricately connected to the redox transitions of the chiral copper catalyst. This collective mechanism tackled the various selectivity issues and eventually yielded one etherified stereoisomer 3. In cases where anomeric unprotected reducing sugars were employed, a dynamic kinetic resolution type glycosylation5a, 32 is further activated to yield synthetically challenging β-O-glucosides and β-O-1,2-cis mannosides 4. Further, our developed regioselective Williamson-type etherification from α-haloamides opens up an important carboxamidation transformation class that is currently underrepresented in site-selective carbohydrate functionalization. Significantly, a broad range of naturally occurring saccharides can be regioselectively functionalized without the cooperative assistance of an organoboron catalyst that was often essential in previously reported strategies.3, 5a
Results and Discussion
Establishment of the Copper Catalyzed Site-selective Etherification
We commenced our investigation by utilizing the minimally protected mannosyl triol 1 a and α-chloroamide 2 a as substrates for our model reaction. In our initial evaluation of a library of chiral bis-oxazolidine (BOX, PyBOX) and bis-phosphine ligands (see Table 1A and the Supporting Information, Table S1), we observed a profound influence of the identity of the ligand with the regioselectivity (r.r.) and the diastereoselectivity (d.r.) outcome.
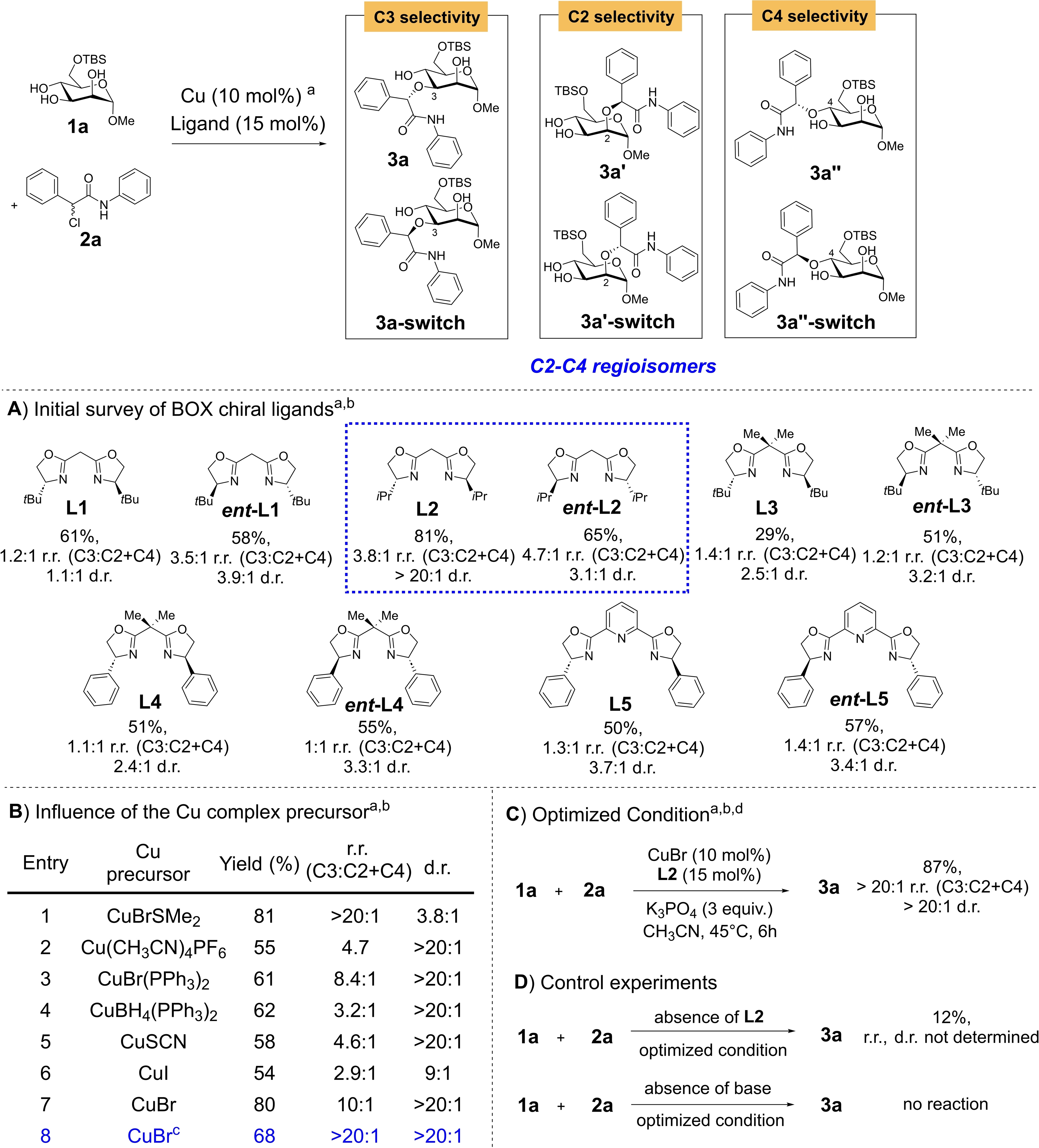
- Conditions: [a] Polyol 1 a (0.1 mmol), 2 a (0.2 mmol), CuBrSMe2 catalyst (10 mol %), ligand (15 mol %), K3PO4 (3.0 equiv.) in acetonitrile (0.05 M with respect to 1 a) at 45 °C for 24 h. For entries involving L1, L2, L3, L4 and L5, 3 a was generated as the major C3 regioisomer, 3 a’ and 3 a’’ were generated as the minor C2 and C4 regioisomers respectively. For entries involving ent-L1, ent-L2, ent-L3, ent-L4 and ent-L5, 3 a–switch was generated as the major C3 regioisomer, 3 a’-switch and 3 a’’-switch were generated as the minor C2 and C4 regioisomers respectively. [b] Yields, d.r. and r.r. (C3:C2+C4) ratio were determined by crude 1H NMR spectra analysis using 1,3,5-trimethoxybenzene as an internal standard. [c] Reaction conducted for 12 h. [d] 2 a (0.3 mmol, 3 equiv.), c=0.1 M instead.
Generally, bis-phosphine ligands reacted poorly in this reaction, while amongst the various BOX ligands tested, only the isopropyl derivative L2 displayed promising regioselectivity (3.8 : 1) and excellent diastereoselectivity. BOX ligands were considered in our optimization as they were known to be robust and compatible with radical copper catalysis broadly in enantioselective protocols.24-26, 33 Chiral catalyst control was also conspicuous as the modification of the ligand to its ent-L2 congener diminished the diastereoselectivity control. Encouraged by these preliminary results, we shortened the reaction time to 24 h and surprisingly noted that the regioselectivity improved although diastereoselectivity worsened in this case (Table 1B, Entry 1). A more all-rounded selectivity outcome was realized when we modified the copper catalyst precursor to CuBr (see Table 1B, Entry 7–8), which generated the C3-selective etherification product 3 a with good yields (68 %) and excellent r.r. and d.r. We noted additionally that meticulous control of reaction time is of utmost essence, and prolonged reaction time was responsible for isomerization side reactions that often resulted in r.r. and d.r. reduction. This supported the postulate of the reversibility of downstream stereocontrolling elementary steps and/or base promoted epimerizations. This was further confirmed when we subjected isolated 3 a to the standard optimized conditions (see Supporting Information Section 9), which yielded a mixture of the epimer 3 a–switch (22 %), the C2-regiomer 3 a’ (13 %) and recovered 3 a (56 %) after 48 h.
Further fine tuning of conditions (see Supporting Information Table S3–S5) such as reaction time, concentration, temperature and solvent led us to our optimized conditions at a considerable shortened reaction time of 6 h, which yielded 87 % of the product 3 a with excellent r.r. and d.r (Table 1C). Further control experiments (Table 1D) also ascertained that ligand L2 and the base were critical in the reactivity of our reaction, as yields were severely diminished in the absence of either of these. We also studied the influence of the less hindered methyl BOX derivative (see Supporting Information Table S7 Entries 9–10, L6 and ent-L6) at the optimized condition, which revealed that r.r. and d.r. were diminished. Considering that the sterically more hindered L1 gave lower selectivities in the initial screening, this suggested that a correct balance of steric hindrance is ingrained within the catalytic mechanism to achieve the most optimal ligand/sugar matching combination.
After attaining the optimal condition, we proceeded to evaluate the robustness of our method across a wide spectrum of stereochemically diverse saccharides (Table 2). First, we examined the protecting group tolerance and stereochemistry modification on the anomeric center within the mannosyl scaffold. Gratifyingly, a range of protecting groups common to carbohydrate chemistry such as TBS (3 a, 3 d–f) and benzyl (Bn, 3 b–c) are readily tolerated in this protocol. It is interesting to note that while O3-functionalized products were generally observed, when O4 was protected in the case of 3 c, the site-selectivity switches to the O2-functionalized product. We posit that O4 protection could pose substantial steric hindrance in close proximity, thereby disfavoring the O3-functionalized product. This phenomenon of regioselectivity switch upon O4-substitution was also previously observed by Niu and co-workers,6 and attributed to steric influences. Both α- and β-anomers of thioglycosides (3 d–e) are also readily assimilated in our strategy to yield the respective etherified products with excellent r.r. and d.r. An alkyne handle can also be installed onto the saccharide (3 f) to incorporate advantages of the well-recognized CuAAC CARBOCLICK strategy34 into our functionalized products.
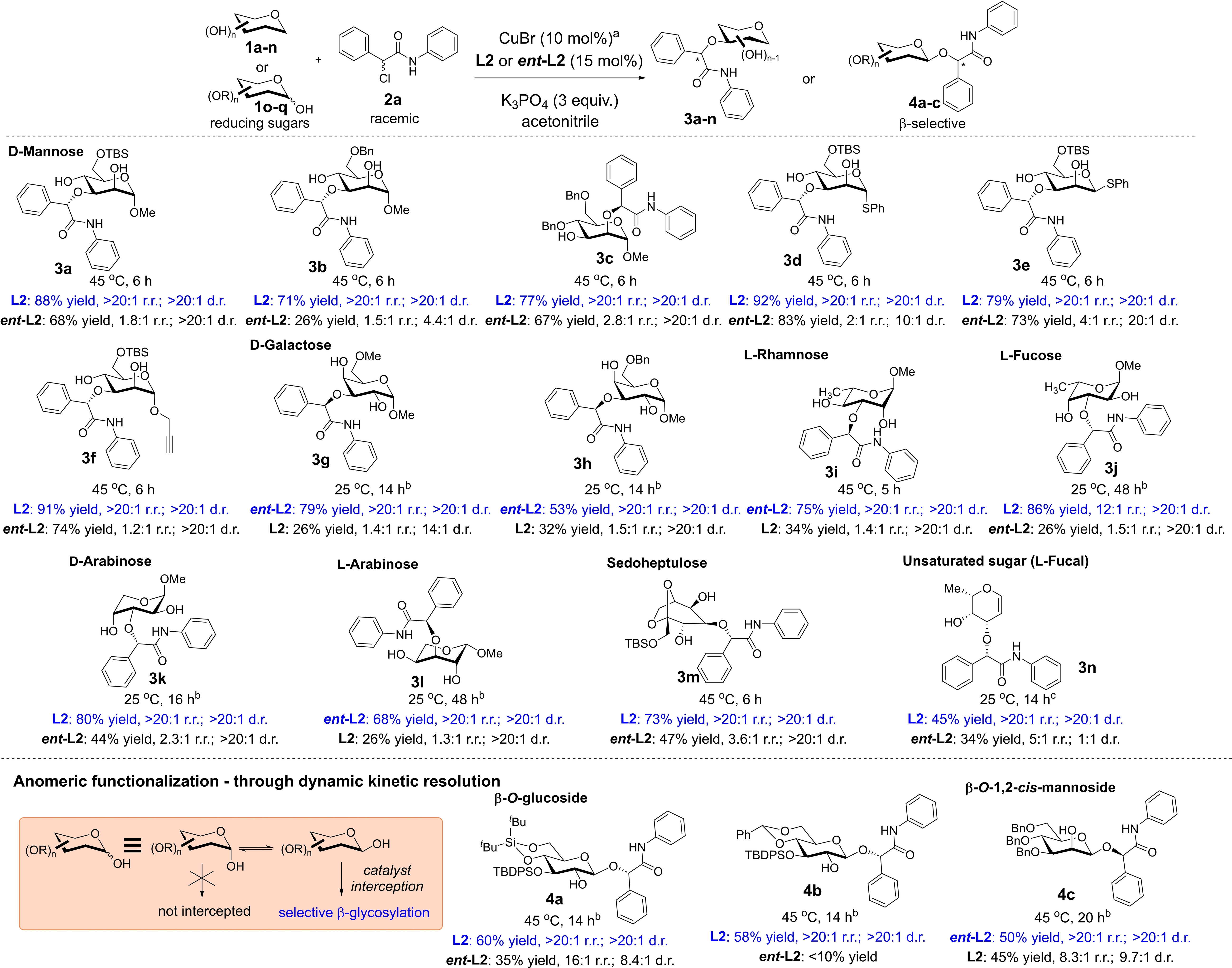
- Conditions: [a] Polyol 1 a–q (0.2 mmol), 2 a (0.6 mmol), CuBr catalyst (10 mol %), L2 or ent-L2 (15 mol %), K3PO4 (3.0 equiv.) in acetonitrile (0.05 M with respect to 1 a), r.r. and d.r. ratio were determined by crude 1H NMR spectra analysis using 1,3,5-trimethoxybenzene as an internal standard; reported yields are isolated yields after silica gel flash column chromatography. [b] 10 mol % TBAC as additive, 2 a (0.3 mmol) instead. [c] CuBr catalyst (20 mol %), L2 (30 mol %), TBAC (20 mol %) instead.
By permutating the identity of the saccharide, we were also delighted that a broad range of monosaccharides performed robustly in our strategy to yield the etherified sugars with excellent regio- and diastereoselectivity. This include naturally occurring hexoses such as d-mannose (3 a–f), d-galactose (3 g–h), l-rhamnose (3 i), l-fucose (3 j); pentoses such as d, L-arabinoses (3 k and 3 l respectively); rare but also naturally occurring heptoses such as sedoheptulose (3 m) can also be well assimilated in this strategy. Useful unsaturated sugars that often serve as critical building blocks, such as l-fucal (3 n) can also be harnessed as substrates using our strategy. A ligand-polyol matched/mismatched effect was also evident (matched conditions marked out in blue). We noticed deleterious effects principally on regioselectivity and yield throughout the scope in the mismatching combinations. This further supports the postulate that the stereochemical information on the catalyst is key in discriminating the multiple hydroxyl groups on the saccharide.
We sought to push the frontier of our strategy by evaluating its effectiveness on anomeric unprotected reducing sugars 1 o–q (Table 2). Selectively functionalizing such substrates are particularly challenging catalytically, as the anomeric position is endowed with a fluctuating hemiacetal scaffold, and thus they exist as a mixture of equilibrating anomers in solution. Significantly, any catalytic strategy that seeks to tackle this class of saccharides for anomeric functionalization would necessitate the overcoming of the fore-mentioned challenge using dynamic kinetic resolution control.5a, 32, 35 While we recognize that reducing sugars could be alkylated stereoselectively in the literature by exploiting classical thermodynamic or kinetic control,36 these were often achieved non-catalytically through judiciously tuned stoichiometric base and temperature modifications,37 or via chelation using additives,38 without concurrent stereogenic center creation. Gratifyingly, our catalytic system tackled the anomeric, site-, and diastereoselectivity issues concurrently. This enabled access into the β-O-glycosides selectively on the anomeric position, with the contemporaneous stereoselective creation of the exogeneous stereogenic center on the α-amidic carbon. Besides showcasing the selective synthesis of β-glucosides 4 a–b, we also successfully accessed the challenging but highly demanded β-1,2-cis mannosyl system in 4 c.39 Interestingly, our strategy differs from previously known organoboron catalyzed methods which generated 1,2-cis glycosides from reducing sugars.5a, 35a Our method on the contrary, appeared to be agnostic towards the relative stereochemistry of the C2-hydroxy group with respect to the anomeric substituent, since β-anomers were consistently generated. Thus, this observation likely precludes the intermediacy of a bidentate sugar-copper chelate analogous to boronate esters, which require a 1,2-cis-vicinal diol scaffold.
We also studied the scope by permutating the α-haloamide substrate (Table 3), and are pleased to find out that a considerable range of electron withdrawing (3 o–q, 3 w, 3 y) and electron donating (3 r–u, 3 x, 3 z) substituents could be readily installed on the aromatic rings at different positions with overall retention of excellent regioselectivity. Upon ligand modification to ent-L1 and switching to the α-bromoamide, aliphatic moieties such as the ethyl group (3 za) is further tolerated to access the etherified product with moderate yield, and very good regioselectivity.

- Conditions: [a] Polyol 1 a (0.2 mmol), 2 b–n (0.6 mmol), CuBr catalyst (10 mol %), L2 or ent-L2 (15 mol %), K3PO4 (3.0 equiv.) in acetonitrile (0.05 M with respect to 1 a), r.r. and d.r. ratio were determined by crude 1H NMR spectra analysis using 1,3,5-trimethoxybenzene as an internal standard; reported yields are isolated yields after silica gel flash column chromatography. [b] 10 mol % TBAC as additive, 2 a (0.3 mmol) instead. [c] CuSMe2 catalyst (10 mol %), ent-L1 (15 mol %), the α-bromoamide derivative used instead.
To ensure a reliable assignment of the absolute configuration of our compounds, we subjected derivative 3 a to vibrational circular dichroism (VCD) analysis (See Supporting Information Section 7).40 After comparing the DFT calculated spectra and the spectroscopically measured Infra-Red (IR) and VCD spectra, the newly generated chirality center on the α-amide carbon can be unambiguously assigned as the (S)-configuration. The site-selectivities of all derivatives were confirmed by hetereonuclear multiple bond correlation (HMBC) NMR spectroscopy, and the configurations of their new stereogenic center were assigned by analogy to 3 a. The β-anomeric selectivity of 4 a–c was also counterchecked by using the diagnostic 1JC-H coupling constant of the anomeric carbon (See Supporting Information Supplementary Note).41
Considering the importance of verifying the radical nature of the chiral catalytic pathway, we executed the following mechanistic investigations. First, we added stoichiometric quantity of the radical scavenger (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) under the standard optimized condition (Figure 2A). In addition to isolating the radical trapped adduct 5, which evidences the presence of the α-C-centered radical subsequent to the homolytic fission of the C−Cl bond, we also observed the complete termination of the reaction without any traces of 3 a detected. This supports the postulate that a radical catalytic pathway is pivotal in our site-selective strategy.
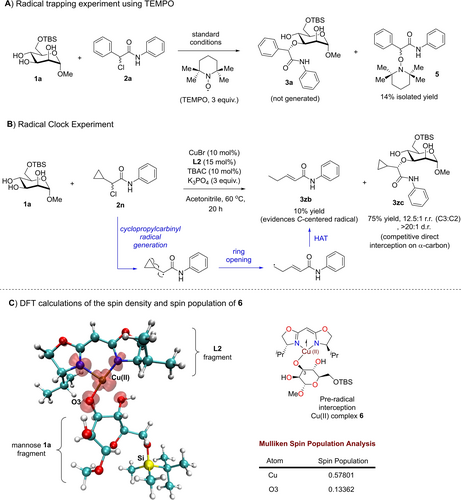
Mechanistic investigations by experiments and theory. (A) Radical trapping control experiment using TEMPO. (B) Radical clock experiment. (C) DFT computed spin density topology and spin populations of 6 at the r2-SCAN3c/CPCM(acetonitrile) level of theory. Red transparent isosurfaces denote the spin density. (Atoms color: Cu brown, Si yellow, C cyan, O red, N blue, H white)
Second, we conducted a radical clock control experiment using the cyclopropyl derivative of the α-chloroamide as a radical probe (Figure 2B). We detected, isolated and fully characterized a ring opening side product 3 zb,26a which evidences the involvement of a cyclopropylcarbinyl42 C-centered radical in the ring opening and a subsequent hydrogen atom transfer (HAT).43 The radical clock control additionally strengthens the existence of a radical catalytic mechanism.
To gain further insights into relevant copper catalytic species, we computed the spin density topology using ORCA44 and Multiwfn45 on the postulated open shell Cu(II)/mannose 1 a radical species 6 prior to the α-radical interception elementary step at the r2-SCAN3c/CPCM(acetonitrile)46 level of theory (Figure 2C). Supplementing the spin density topology with a Mulliken spin population analysis,45 we noted the highest residence of alpha spin population on the copper center (0.57801, spin population largely residing in the more diffused d-orbitals) rather than the C3-hydroxyl oxygen (0.13362, spin population largely residing in the more compact p-orbitals), which is consistent with calculations by Fu and co-workers.25 Therefore, this offers good basis that the carbon-centered α-amide organic radical would more likely undergo homolytic fusion directly with the Cu(II) core instead of the O3 oxygen to form the Cu−C bond, and thereafter to yield a high valent Cu(III) species.
Conglomerating our data and previous literature knowledge in chiral copper radical catalysis,25 we propose the following one electron based Cu(I)/Cu(II)/Cu(III) mechanistic hypothesis that best fits our observations (Figure 3A). The CuBr precursor first undergoes ligand exchange, plausibly with BOX ligand L2 and the acetonitrile solvent to generate the deprotonated catalytic Cu(I) species 7. A subsequent single electron transfer (SET) process of 7 with α-chloroamide 2 a generates the Cu(II) catalytic species 8 and the organic radical R ⋅ . This process is supported by our radical trapping and radical clock control experiments.
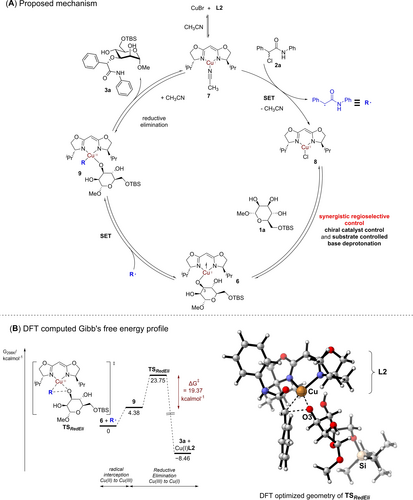
Proposed mechanism and DFT computed Gibb's free energy profile. (A) Proposed catalytic radical mechanism of the site-selective carbohydrate functionalization. (B) Gibb's free energy profile (left) of the feasible O3-regioselective functionalization computed at the PWPB95-D4/def2-QZVPP/CPCM(acetonitrile)//r2-SCAN3c/CPCM(acetonitrile) level of theory. CYLView render (right) of the DFT computed transition state TSRedEli is provided.
As our O3-polyol regioselectivity preference appeared to parallel observations in regioselective arylations made by Niu,6 as well as earlier investigations studying the intricate relationship between pKa, reactivity and nucleophilicity at various positions,47 we posit that stereoelectronic effects are likely crucial in a subsequent base promoted regioselective deprotonation elementary step to access the open-shell Cu(II) species 6. The importance of the base deprotonation elementary step is also evidenced by our control experiment (Table 1D), where the absence of base resulted in no product generated. It is also vital in this discussion to appreciate literature known subtle and complex reactivity differences between the different hydroxyl groups on the carbohydrate polyol substrate.48 The equatorial configuration of a hydroxyl group is often preferred, as axial functionalization would result in unfavorable 1,3-diaxial interactions.47b, 49 Further, intramolecular hydrogen bonding within the saccharide ring are known to culminate in counter-intuitive reactivity preferences.2g, 50 We also conducted further control experiments using a 6-OH unprotected mannosyl tetraol 1 n (see SI, Section 10), and observed a similar observation as our recent work,5b that freeing the 6-OH group has marked deleterious effects on reactivity (18 % yield of the 3-OH functionalized product). This observation is likely due to the establishment of unproductive intramolecular hydrogen bonds within the saccharide, that attenuates reactivity despite the intuitively higher nucleophilicity of a sterically less hindered primary alcohol.
Upon the profound appreciation that known stereoelectronic, substrate effect and hydrogen bonding influences would collectively be in operation, we clearly emphasize that chiral catalyst control is concurrently pivotal, as enantiomeric identity of the chiral ligand shows a clear matched/mismatched influence on the regioselectivity (see Supporting Information Section 4, Tables S8 and S9). Our data thus collectively suggested that the origin of the regioselectivity is governed by a balanced combination of catalyst and substrate control.
Subsequently, we reasoned that R ⋅ would be preferentially intercepted by the Cu(II) core in 6 due to its high alpha spin population in a second SET process to yield the Cu(III) species 9, which sets the stage for the stereoselective C−O bond forming step. A reductive elimination would eventually generate the etherified sugar 3 a. Upon ligand exchange, a concomitant regeneration of the Cu(I) species 7 would then restart a new catalytic cycle. We further explored the feasibility of this working hypothesis by modelling the Gibb's free energy profile of the radical interception and the following reductive elimination steps at the PWPB95-D4/def2-QZVPP/CPCM(acetonitrile)//r2-SCAN3c/CPCM(acetonitrile) level of theory (Figure 3B).46b, 51 Gratifyingly, we located the transition state for the reductive elimination TSRedEli, and computed a kinetically feasible Gibb's free energy barrier of 19.37 kcalmol−1. Furthermore, the overall exergonicity of the reaction (−8.46 kcal mol−1) also constitutes a favorable thermodynamic driving force toward 3 a.
Last but not least, we demonstrated the synthetic utility of our strategy in accessing disaccharides (Figure 4). First, we executed a facile TBAF deprotection of the O6-TBS group which generated mannosyl-triol 10 in very good yields (Figure 4A). This is followed up by a regioselective 2-deoxyglycosylation52 with a galactal under acid catalysis to provide α-(1→6) linked 2-deoxyglycoside 12. In view of overwhelming reports in the last decade documenting the requirement of employing heavily protected 6-OH unblocked glycosyl acceptors in Brønsted acid catalyzed 2-deoxyglycosylation,19, 53 our route offered an instructive instance how a minimally protected mannoside can be consecutively functionalized at the O3 and O6 position within a short synthetic design. By virtue of the broadly recognized usefulness of CLICK chemistry in the glycosciences to link saccharide motifs for applications such as bioorthogonal coupling and drug development,34 we successfully subjected alkyne derivative 3 f to a copper catalyzed CuAAC CARBOCLICK strategy which facilitated access to synthetic glycosides 14 bearing the carboamide functionalization with excellent yields (Figure 4B). Further, in light of the presence of the 2-aminoglycoside motif in 3-carboxamide bearing amphetamine prodrugs,28 we first executed a 4,6-benzylidine protection of 10 to form 16 in good yields (Figure 4C). Next, we carried out a serial azidation/reduction sequence without intermediate purification of azide 17 to gain direct entry into the biologically relevant 2-amino-3-carboxamide privileged scaffold54 in 18.28
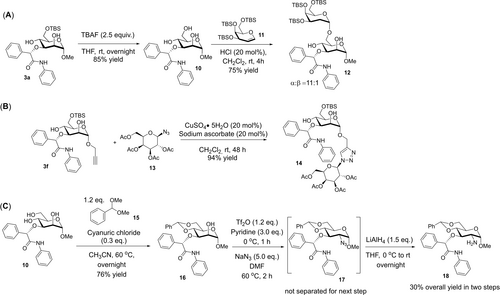
Further derivatizations and synthesis of diverse disaccharides. (A) A regioselective synthesis of an α-(1→6) glycosidic linkage to access O3,O6-difunctionalized mannose. (B) A CuAAC CARBOCLICK functionalization on an etherified mannose endowed with an alkyne handle. (C) Derivatization to access the biologically relevant 2-amino-3-carboxamide scaffold.
Conclusions
To sum, we disclose a seminal case of an enantioconvergent site-selective carbohydrate etherification that proceeds via a chiral copper catalyzed radical mechanism. This instance introduces the emerging utility of chiral radical catalysis into the broad field of selective carbohydrate synthesis. Intriguingly, we demonstrate that the asymmetric radical catalytic pathway tackles multiple dimensions of stereoselectivity, which include aspects of enantioconvergence, site-selectivity, chirality generation and diastereoselectivity. Further, difficult substrates such as anomeric unprotected reducing sugars were amenable, and unlocked an additional facet of dynamic kinetic resolution stereocontrol at the anomeric center to yield the more challenging β-O-glycosides, such as the highly demanded β-O-1,2-cis mannoside selectively. Further investigations to develop chiral radical catalysis in carbohydrate synthesis are underway in our laboratories.
Acknowledgments
Both C. C. J. L. and C. M. thank the Boehringer Ingelheim Foundation for the very generous funding support through the Plus 3 Perspectives Programme. C. M. acknowledges funding from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy (EXC-2033; project no. 390677874) and the DFG's Heisenberg programme (ME 4267/5-1; project no. 418661145). We further acknowledge Fonds der Chemischen Industrie (VCI) for generous funding to C. C. J. L. through a Liebig fellowship (Li 198/04). Prof. Herbert Waldmann is greatly acknowledged for his generous assistance and infrastructural support. H. G. acknowledges the Boehringer Ingelheim Foundation for postdoctoral funding. We are grateful for infrastructural support from the Technical University of Dortmund and the Max Planck Institut für Molekulare Physiologie, Dortmund. Open Access funding enabled and organized by Projekt DEAL.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




