Acyclic Cucurbit[n]uril Receptors Function as Solid State Sequestrants for Organic Micropollutants
Abstract
The accumulation of organic micropollutants (OMP) in aquatic systems is a major societal problem that can be addressed by approaches including nanofiltration, flocculation, reverse osmosis and adsorptive methods using insoluble materials (e.g. activated carbon, MOFs, nanocomposites). More recently, polymeric versions of supramolecular hosts (e.g. cyclodextrins, calixarenes, pillararenes) have been investigated as OMP sequestrants. Herein, we report our study of the use of water insoluble dimethylcatechol walled acyclic cucurbit[n]uril (CB[n]) hosts as solid state sequestrants for a panel of five OMPs. A series of hosts (H1–H4) were synthesized by reaction of glycoluril oligomer (monomer–tetramer) with 3,6-dimethylcatechol and fully characterized by spectroscopic means and x-ray crystallography. The solid hosts sequester OMPs from water with removal efficiencies exceeding 90 % in some cases. The removal efficiencies of the new hosts parallel the known molecular recognition properties of analogous water soluble acyclic CB[n]. OMP uptake by solid host occurs rapidly (≈120 seconds). Head-to-head comparison with CB[6] in batch-mode separation and DARCO activated carbon in flow-through separation mode show that tetramer derived host (H4) performs very well under identical conditions. The work establishes insoluble acyclic CB[n]-type receptors as a promising new platform for OMP sequestration.
Introduction
Throughout the 20th century, the power of synthetic organic chemistry has resulted in the creation of numerous compounds that improved the lives of humanity. Unfortunately, some of these compounds have high potential to persist in the earth's water systems when improperly used, disposed of, or discharged. Industrial dyes, pharmaceuticals, personal care products, herbicides, pesticides, and industrial chemicals constitute organic micropollutants (OMP) of high concern that result in significant health and environmental issues.1 For example, bisphenol A (BPA) and bisphenol S (BPS) are widely used chemicals in plastic manufacturing that slowly leach into the environment (Figure 1a).2 BPA and BPS have been detected in water sources worldwide and in numerous aquatic species.3 Perfluoroalkyl substances (PFAS, e.g. perfluorooctanesulfonate (PFOS) and perfluorooctanoic acid (PFOA)) are part of a class of mass produced chemicals that can persist in environment for several decades. Due to their long serum half life in human (4–5 years,)2 they readily bioaccumulate in humans and cause adverse health effects in the liver, thyroid and kidney.4 Finally, methyl violet (MV) is another OMP commonly used in the textile, cosmetics, and leather industries where it can easily contaminate water sources. MV is a well known mutagen that can cause cancer, allergies, and kidney diseases.5 A variety of methods have been investigated for the removal of OMPs from water sources including coagulation, flocculation, micro- and nanofiltration, reverse osmosis, chemical oxidation, photodegradation, and adsorption.5b Adsorptive methods are the most popular due to their simplicity and efficiency and a variety of materials (e.g. activated carbon, bioadsorbants, nanocomposites, metal–organic frameworks, polymers, metal oxides) have been investigated and reported in the literature.6 Activated carbon is the most widely employed adsorptive separation material, but suffers from poor uptake of polar compounds and energy intensive and complex regeneration processes.7
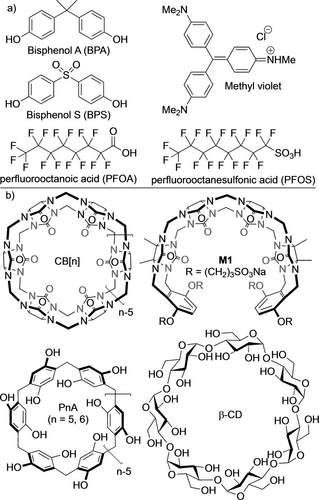
a) Chemical structures of OMPs used in this paper. b) Selection of macrocyclic molecular containers used as sequestrants.
In recent years, a variety of non-conventional polymeric materials based on supramolecular hosts have been investigated for the removal of OMPs from water. In work that has inspired the field, Dichtel and co-workers reported the synthesis of a variety of β-cyclodextrin (Figure 1b) polymers, which exhibit the rapid removal of a wide variety of OMPs from water.7, 8 Subsequently, a variety of macrocyclic host systems have been investigated for OMP removal including calixarenes and calixpyrroles, pillararenes, cavitands, and naphthotubes.6, 9 Macrocycles have also been used to address related separations challenges in solution and with non-porous adaptive crystals.9c, 10
Our group has a long-standing interest in the synthesis and molecular recognition properties of macrocyclic cucurbit[n]uril (CB[n]) hosts (Figure 1b).11 Within the scope of macrocyclic CB[n], the groups of Buschmann and Jekel previously studied the ability of CB[6] to remove reactive dyes from textile waste streams, whereas Khashab and co-workers used CB[7] to separate the isomers of xylene.12 Xiao, Isaacs, and co-workers reported the ability of macrocyclic ns-CB[10] to remove pyridine from toluene/pyridine mixtures.13 We, and others,14 have also synthesized and studied a wide variety of acyclic CB[n] (e.g. M1) that possess good water solubility and high biocompatibility and maintain the essential molecular recognition properties of macrocyclic CB[n] (e.g. high binding affinity in water). These acyclic CB[n] function as in vivo sequestrants for neuromuscular blockers, anesthetics, and drugs of abuse.15 Stuctural modification of acyclic CB[n] is straight forward given that they are prepared from a series of building blocks (glycoluril oligomer, aromatic wall, solubility determining groups). Accordingly, we wondered whether the stuctures of acyclic CB[n] could be tailored to function as solid state sequestrants for OMPs. This paper reports our studies of water insoluble catechol walled acyclic CB[n] as sequestrants for that application.
Results and Discussion
This section is subdivided into sections as follows. First, we report the synthesis of four new water-insoluble acyclic CB[n] hosts (H1–H4) of different glycoluril oligomer length (monomer–tetramer). Next, we study the effect of the oligomer length on the removal efficiency of BPA. Subsequently, we investigate the removal efficiency of five OMPs (Figure 1a) by H4 (Scheme 1) using CB[6] as an active comparator. Thereafter, we show that H4 can be regenerated over at least five cycles without significantly decreasing removal efficiency. Finally, we conducted flow-through experiments and compared them with activated charcoal (DARCOTM) to demonstrate the ability to use H4 in water purification.
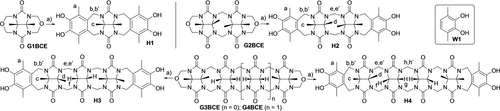
Synthesis of water insoluble acyclic CB[n] hosts H1–H4. Conditions: a) TFA, W1, RT, 33–56 %.
Design and Synthesis of Water Insoluble Catechol Walled Hosts (H1–H4)
Previously, we have studied the molecular recognition properties of acyclic CB[n]-type hosts (e.g. M1) in aqueous solution as a function of the glycoluril oligomer length and the nature of the aromatic sidewalls and solubilizing groups.11b We found that glycoluril tetramer derived hosts (e.g. M1) are more potent receptors than those derived from shorter glycoluril oligomers (e.g. monomer–trimer) due the more fully formed ureidyl carbonyl portals which engage in ion-dipole interactions and that sulfonate groups enhance aqueous solubility as well as binding affinity by secondary ion-ion interactions with cationic guests.16 To prepare new hosts that are well suited as solid state sequestrants for OMPs, we choose to remove the (CH2)3SO3Na solubilizing groups and leave the OH substituents which dramatically decrease aqueous solubility. New hosts H1–H4 (Scheme 1) differ in the length of the glycoluril oligomer backbone. For the synthesis of H1–H4, we employed our building block method11b, 17 involving the double electrophilic aromatic substitution reaction of a glycoluril oligomer bis(cyclic)ether (G1BCE–G4BCE) with activated dimethyl catechol wall W118 in TFA at room temperature (Scheme 1). Hosts H1–H4 were obtained in 56, 33, 55, and 43 % yield, respectively, after washings and recrystallization. Hosts H1–H4 are very poorly soluble in all common solvents including hot DMSO which necessitated the use of TFA as the NMR solvent. Each new host was fully characterized by 1H NMR, 13C NMR, IR, and mass spectrometry (Supporting Information). The spectroscopic data recorded for H1–H4 is in accord with the depicted C2v-symmetric structures. For example, the 1H NMR spectra recorded for H1–H4 in TFA (Figure 2) with a D2O capillary tube for locking show the expected number of methyl (H1: 2; H2: 3; H3: 3; H4: 3), diastereotopic methylene (H1: 2; H2: 4; H3: 4; H4: 6), and glycoluril methine (H1: 0; H2: 0; H3: 1; H4: 2) resonances. Similarly, the number of resonances in the 13C NMR spectra recorded for H1 (8 observed, 8 expected), H2 (11 observed, 11 expected), H3 (13 observed, 13 expected), and H4 (15 observed, 15 expected) are consistent with C2v-symmetry.
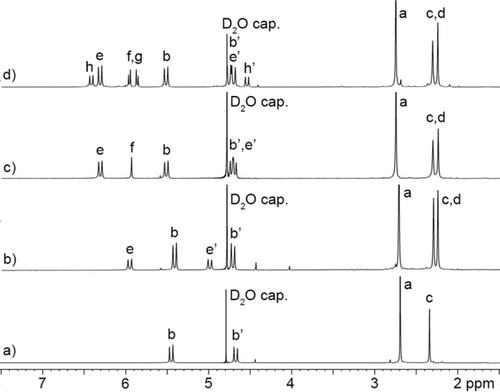
1H NMR spectra recorded (400 MHz, TFA, D2O capillary, RT) for: a) H1, b) H2, c) H3, d) H4.
X-ray Crystal Structures of H3 and H4
We were fortunate to obtain single crystals of H3 and H4 upon recrystallization from TFA/H2O and TFA/MeOH, respectively, and their structures were solved by x-ray crystallography. Figure 3a shows a cross eyed stereoview of the structure of H3 in the crystal (CCDC 2323728). Similar to other hosts based on glycoluril trimer17b H3 is C-shaped. The angle between the mean planes of the aromatic walls amounts to 72.5°. The most striking feature of the structure is the presence of a molecule of H2O which accepts two H-bonds from the hydroxyl groups on the tips of the aromatic walls. The O−H⋅⋅⋅O angle is 174.768° whereas the O⋅⋅⋅O and H⋅⋅⋅O distances are 2.676 and 1.839 Å. The structural constraint of these two H-bonds results in a slight cupping of the molecule. For this reason, two different distances are measured between the opposing methylene groups (e.g. H2C⋅⋅⋅CH2; 10.940 and 9.658 Å). For comparison, the analogous distance for CB[6] is 9.585–9.916 Å which indicates that the cavity of H3 is similar in size to CB[6].19 Molecules of H3 pack in the crystal by offset π–π stacking between the external faces of the aromatic sidewalls to form tapes along the y-axis as shown in Figure 3b. The separation between the mean planes of the aromatic walls of adjacent molecules of H3 is 3.4059 Å. In the offset π-stack geometry one Ar-CH3 group sits 3.529 Å above the centroid of the opposing aromatic wall and vice versa. The tapes pack with their long-axes parallel along the y-axis.
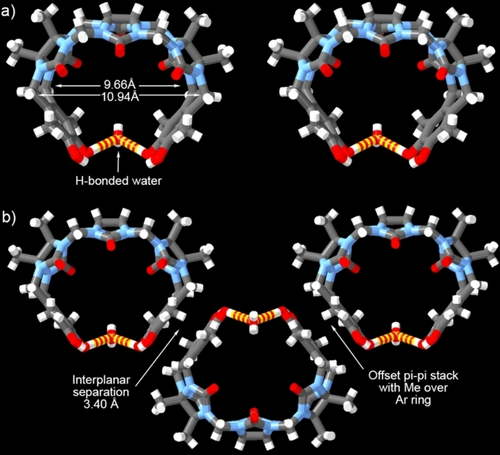
a) Cross-eyed stereoview of a molecule of H3 in the crystal. b) Packing of three molecules of H3 along the y-axis. Color code: C, gray; H, white; N, blue; O, red; H-bonds, yellow-red striped.
We also obtained single crystals of H4 (CCDC 2323729) and determined its structure by x-ray crystallography (Figure 4). The cavity of H4 is filled by a molecule of trifluoroacetic acid (TFA). The TFA molecule forms a hydrogen bond to one of the ureidyl carbonyl O-atoms (O−H⋅⋅⋅O=C distance, 1.826 Å; O⋅⋅⋅O=C distance, 2.664 Å; O−H⋅⋅⋅O angle, 173.713°). One of the Ar-OH groups forms an intramolecular H-bond with the hydroxyl group on the opposing sidewall (O−H⋅⋅⋅O distance, 2.065 Å; O⋅⋅⋅O distance, 2.782 Å; O−H⋅⋅⋅O angle, 143.010°) to close the cavity. The glycoluril tetramer backbone of H4 expands its cavity and increases the distances between the opposing CH2 groups (10.771 and 11.251 Å, CH2 groups marked with @ in Figure 4a) compared to the corresponding values for H3. The angle between the mean plane of the aromatic sidewalls of H4 dramatically increases to 114.725° relative to H3. Similarly, the distance between the equatorial quaternary C-atoms increase to 12.583 Å (marked with $ in Figure 4a). All these metrics show that the cavity of H4 is large enough to accommodate a range of OMPs as guests. In the crystal, molecules of H4 pack as tapes along the x-axis driven by the formation of offset π-stacks between the exterior surface of the sidewalls (Figure 4b). The separation between the mean planes of the aromatic sidewalls is 3.4308 Å. In the case of H4, however, it is the o-xylylene CH2 groups that form C−H⋅⋅⋅π interactions with the opposing sidewall.20 The tapes align their long axes parallel to one another and are held together by intra-tape O−H⋅⋅⋅O=C H-bonds (O⋅⋅⋅O distance, 2.857 Å; H⋅⋅⋅O−C distance, 2.068 Å; O−H⋅⋅⋅O angle, 156.093°). These intermolecular H-bonds in the solid state are probably responsible for the very poor solubility of H4 in water (3.4 μM, Supporting Information). For comparison, the solubility of CB[6] (6.5 μM) and CB[8] (10 μM) are somewhat higher.21 The solubility of H4 in water was determined by incubating excess H4 (28 mg) in high performance liquid chromatography (HPLC) grade water (50 mL) for 5 hours. The resulting heterogeneous mixture was centrifuged and filtered through a syringe filter to remove the residual solid. The filtrate was concentrated and the concentration of H4 was determined using 1H NMR in the presence of a known concentration of hexamethyl p-xylenediammonium dibromide as solubilizing guest. The poor water solubility of H4 enhances its function as a solid state sequestrant (see below).
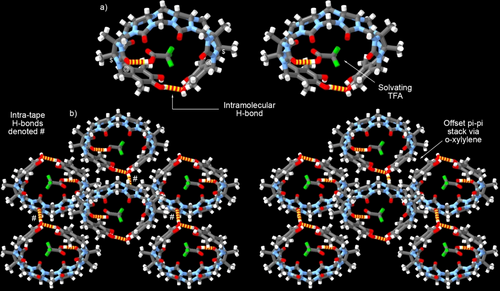
a) Cross-eyed stereoview of a molecule of H4 in the crystal. b) Tape like packing of H4 along the y-axis. Color code: C, grey; H, white; N, blue; O, red; F, green; H-bonds, yellow-red stripped.
Influence of Glycoluril Oligomer Length on the Efficiency of Solid State Sequestration of BPA using H1–H4
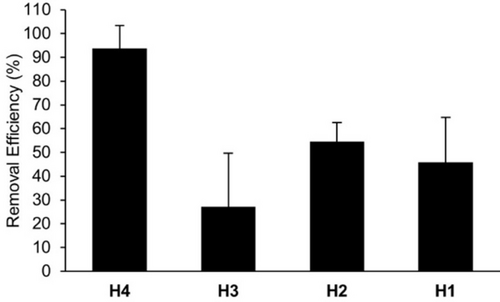
Plot of removal efficiency of BPA (240 μM, 1 mL) from aqueous solution after incubating with solid H1–H4 (9.7 μmoles; H1, 4.8 mg; H2, 6.7 mg; H3, 8.3 mg, H4, 10.0 mg) for 2 h as determined by HPLC of the supernatant after sequestration. Experiments were performed in triplicate (n=3). Error bars represent the error propagation of uncertainty.
Efficiency of Sequestration of the Five OMPs using H4
Next, we investigated the ability of H4 as a solid state sequestrant for the five different OMPs (two neutral, two anionic, 1 cationic). Given that CB[6] has previously been investigated as a sorbent to remove reactive dyes from aqueous textile industry waste streams,12a-12c we elected to use CB[6] as an active comparator. Because the cavity of H4 is larger (Figure 4, $⋅⋅⋅$=12.583 Å) and more flexible than CB[6] (analogous C⋅⋅⋅C distance=10.203 Å),19 we also included CB[8] (analogous C⋅⋅⋅C distance=12.650 Å)25 as an additional comparator. Please note that the quoted distances are C⋅⋅⋅C distances and exclude the van der Waals radii of the specified atoms which are often taken into account when calculating precise cavity volumes.26 Experimentally, we incubated solid H4 (9.7 μmol, 10.0 mg), CB[6] (9.7 μmol, 9.6 mg), or CB[8] (9.7 μmol, 13.0 mg) with each of the five OMPs for 2 hours at room temperature using a ThermoMixerTM (1000 rpm). After centrifugation, the concentration of OMP remaining in solution was determined by HPLC (BPA, BPS, PFOA, PFOS) or UV/Vis (MV) assays (Supporting Information) and the removal efficiency values were determined using eq. 1. The results are shown in Figure 6. Interestingly, acyclic CB[n]-type host H4 is a significantly more effective solid state sequestrant than CB[6] for the panel of OMPs selected. H4 functions well as a sequestrant for BPA (93 %), BPS (83 %), and MV (91 %) and less well as a sequestrant for anionic OMPs PFOS (47 %) and PFOA (33 %). Given that the cavity of H4 may be too large for the narrow cross section of PFOA and PFOS we also performed the sequestration using the smaller H3 host. We found that PFOA (22 %) and PFOS (19 %) are even less well sequestered with the smaller H3 host. These trends can be explained based on the molecular recognition properties of the different hosts (H4, CB[6]). For example, PFOA and PFOS are anionic at neutral pH in water but macrocyclic CB[n] and acyclic CB[n]-type receptors are known to discriminate against anionic guest due to unfavorable anion-dipole interactions.27 Conversely, cationic dye MV is efficiently removed by H4 due to favorable cation-dipole interactions that draws the OMP into the H4 solid. H4 performs better than CB[6] at the removal of MV probably due to its ability to flex and expand its acyclic cavity to accommodate the large MV guest which cannot become encapsulated inside the more rigid CB[6] host. H4 is also more effective at removing the neutral OMPs BPA and BPS from water than CB[6]. Water soluble acyclic CB[n] based on glycoluril tetramer are known to bind to p-phenylene derivatives16a, 28 whereas the p-phenylene derivatives bind less strongly to CB[6] due to steric clashes in the inclusion complexes.27a, 29 Since anionic OMP (PFOA and PFOS) uptake by both H4 and CB[6] was inefficient, the OMP uptake of CB[8] was only studied with BPA, BPS, and MV. As shown in Figure 6, CB[8] functions as well as H4 as a sequestrant for BPA (93 %) but significantly worse for BPS (22 %). We surmise that the SO2 moiety of BPS is more highly hydrated than the CH2-group of BPA which reduces BPS uptake. The uptake behavior of CB[8] toward MV is instructive. Rather than CB[8] drawing MV into the solid state, the MV brings CB[8] into water as shown by 1H NMR spectroscopy of the supernatant (Supporting Information, Figure S16). Interestingly, the UV/Vis spectrum of the soluble mixture of CB[8] and MV shows a hypsochromic shift in the λmax value from 584 nm to 542 nm due to complexation (Supporting Information, Figure S15). Overall, these results show that the solution phase molecular recognition properties of acyclic CB[n] host can be used to predict their solid state sequestration behavior.
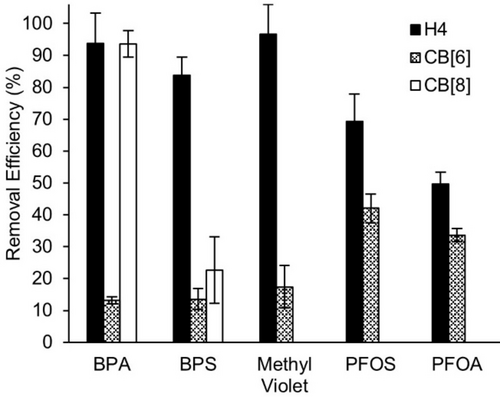
Plots of removal efficiency of OMPs (240 μM, 1 mL) from water after incubating with equimolar (9.7 μmoles) amounts of H4 (10.0 mg), CB[6] (9.6 mg), or CB[8] (13.0 mg) for 2 h at room temperature as determined by UV/Vis (MV) or HPLC (others) of the supernatant. Experiments were performed in triplicate (n=3). Error bars represent the error propagation of uncertainty.
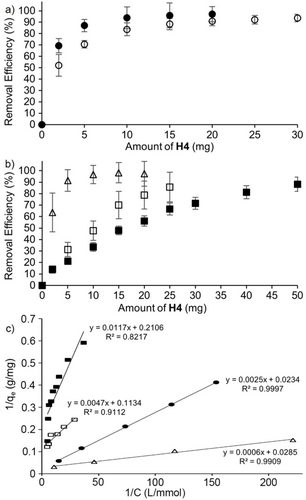
a,b) Plots of removal efficiency of OMPs (240 μM, 1 mL) from water after incubating with different amounts of H4 for 2 h at room temperature as determined by UV/Vis (MV) or HPLC of the supernatant. c) Plot of 1/qe versus 1/C fitted according to the Langmuir isotherm. Key: MV (Δ), BPA (•), BPS (○), PFOS (□), PFOA (▪). Experiments were performed in triplicate (n=3). Error bars represent the error propagation of uncertainty.
Regeneration and Reuse of H4 as a Solid State Sequestrant for BPA, BPS, and MV
Encouraged by the high removal efficiency of H4 for batch-mode purification, we decided to investigate whether the solid consisting of H4 and OMP could be regenerated and reused for further cycles of OMP separation. For these experiments, we selected BPA, BPS, and MV which are most efficiently removed by H4. Experimentally, we used H4 (10 mg) to perform the sequestration of OMP (240 μM, 1 mL) as described above. After centrifugation (11,000 rpm, 10 min.), the supernatant was removed and analyzed. Given that CB[n]•guest complexation in water is driven in part by the hydrophobic effect,30 we investigated the decomplexation of the H4•OMP complexes by washing with organic solvent to regenerate H4. The H4•OMP pellet was suspended in MeOH (1 mL) and shaken/incubated at 50 °C for 20 minutes followed by centrifugation and removal of the supernatant; this process was repeated once. Finally, the H4 pellet was suspended in water (1 mL) at 25 °C and agitated using the ThermoMixerTM for 20 minutes followed by centrifugation and removal of the supernatant; this water washing process was repeated once more. The H4 pellet thus obtained was resubmitted for another sequestration cycle. Figure 8 shows the results of these regeneration and reuse experiments over five cycles. The removal of OMP during the regeneration process after each cycle was confirmed by 1H NMR of the regenerated H4 in TFA where it is soluble (Supporting Information, Figures S23–S25). The data clearly shows that H4 can be regenerated and reused without appreciable loss of removal efficiency which bodes well for the further use of insoluble acyclic CB[n]-type hosts as solid state sequestrants.
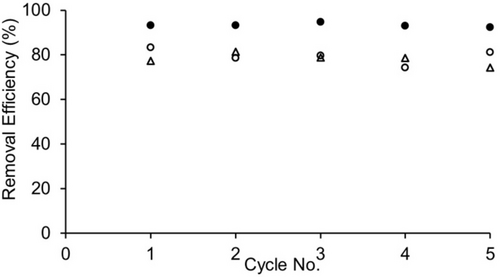
Plot of removal efficiency versus cycle number for the use of solid H4 (10 mg) as a sequestrant for BPA, BPS, and MV from water. After each cycle, uncomplexed solid H4 was regenerated by sequential washing with methanol (50 °C, twice) and then water (25 °C, twice). Experiments were conducted in duplicate (n=2). The data point represents the average of the measurements.
Time Course of the Removal of OMPs from Water using H4
Another critical parameter in the use of solid state sequestrants for batch mode water purification is the amount of time required per cycle. Accordingly, we decided to determine the removal efficiency of solid H4 (10 mg) at a series of different time points (20, 40, 60, 120, 300 seconds) for each of the five OMPs. The results are shown in Figure 9a (BPA, BPS) and Figure 9b (MV, PFOA, PFOS). Obviously, the uptake of the OMPs occurs very rapidly for all five OMPs. The removal efficiency values achieved at 120 seconds are quite comparable to those seen after 2 hours (data in Figures 6 and 7). In comparison, Dichtel reported that the removal efficiency of BPA reaches 95 % in 10 seconds for his β-cyclodextrin-tetrafluoroterephthalonitrile co-polymer system.7 Given the expected differences between molecular and polymeric sequestrants, we consider the performance of H4 for BPA removal to be very good and amenable to further optimization. As expected, our neutral H4 performs significantly less well as a sequestrant for anionic PFOA than the cationic diamondoid porous organic polymer reported by the Ma group.4c
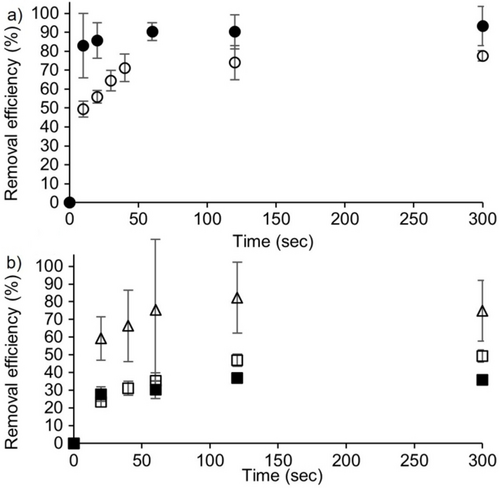
Plots of removal efficiency of OMPs (240 μM, 1 mL) from water as a function of time after incubating with H4 (10 mg) at room temperature as determined by UV/Vis (MV) or HPLC (others) of the supernatant. Key: MV (Δ), BPA (•), BPS (○), PFOS (□), PFOA (▪). Experiments were performed in triplicate (n=3). Error bars represent the error propagation of uncertainty.
Efficiency of OMP Removal in a Flow Through System: Comparison of H4 with DARCOTM
Household water purification filters function in a flow through rather than a batch mode setup and typically employ activated carbon as a stationary phase. Accordingly, we decided to test the removal efficiency of H4 versus activated charcoal (DARCOTM) using a flow through setup. For this purpose, we suspended 20 mg of H4 or DARCO activated carbon in ≈3 mL water to create a slurry. The slurry was forced over an AcrodiscTM (0.45 mm polyethersulfone membrane) 32 mm syringe filter using a syringe to create membranes of H4 or DARCO activated carbon. Subsequently, the OMP (BPA, BPS, or MV) solution (240 μM; 5 mL) was pumped through the membrane using a syringe pump (10 mL/min, 30 s). The filtrate was analyzed by UV/Vis spectroscopy to determine the concentration of OMP and the removal efficiencies. Figure 10 shows a plot of the removal efficiencies for H4 and DARCO activated carbon for each of the OMPs. Under identical conditions, the insoluble acyclic CB[n] host H4 more efficiently removes BPA, BPS, and MV than DARCO which is widely used in real world applications. With an eye toward potential larger scale use of H4 we note that glycoluril oligomer building block G4BCE has been previously prepared by us on a 76 gram scale by a 4-step process31 and that W1 was prepared on a 9.6 gram scale using the two step process reported in the literature.18
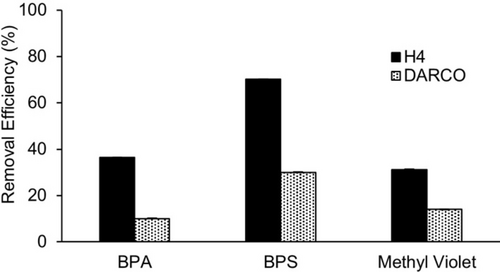
Bar graph of the removal efficiencies measured for BPA, BPS, and MV using solid H4 or activated charcoal (DARCOTM) as a stationary phase supported in a syringe filter with a polyethersulfone membrane. Measurements were performed in triplicate (n=3) and error bars (not visible) represent the standard deviation.
Conclusion
In summary, we have synthesized a series of acyclic CB[n]-type receptors with dimethyl catechol walls that are very poorly soluble in water. The C-shaped structures of H1–H4 were established by 1H and 13C NMR, mass spectrometry, and x-ray crystallography (H3 and H4). Hosts H1–H4 act as solid state sequestrants for OMPs from water with maximal removal efficiencies exceeding 90 % in some cases. The inherent molecular recognition properties of acyclic CB[n]-type hosts (e.g. selectivity for cationic over neutral over anionic guest) translate to the removal efficiencies observed for H4. We find that H4 is the most efficient sequestrant for the OMP panel due to its larger cavity and its more fully formed ureidyl carbonyl portals which promotes interactions with guest. Head-to-head comparison between H4, CB[6], and CB[8] shows that H4 achieves higher removal efficiencies toward all the OMPs studied. Kinetic studies showed that OMP uptake by H4 is rapid with removal efficiencies reaching plateau levels within 120 seconds. In addition, H4 can be recycled and reused at least five times with little loss of efficacy. Finally, we investigated the use of solid H4 as a sequestrant for BPA, BPS, and MV in a flow-through setup and compared the results with those obtained using activated charcoal (DARCOTM) under identical conditions. In conclusion, the work establishes insoluble acyclic CB[n]-type receptors as a promising platform for the development of novel materials for the sequestration of OMPs.
Supporting Information
The authors have cited additional references within the Supporting Information.18, 32
Acknowledgments
We thank the US National Science Foundation (CHE-1807486) for financial support. S.P. thanks the University of Maryland for the G. Forrest Woods Fellowship and the Charlotte Kraebel PhD'59 Endowed Award in Organic Chemistry.
Conflict of interests
L.I. holds equity in Clear Scientific (Cambridge, MA) and is co-founder and holds equity in Reversal Therapeutics (National Harbor, MD).
Open Research
Data Availability Statement
The data that support the findings of this study are presented in the Supporting Information. Supplementary crystallographic data for this paper are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlesruhe Access Structures service.33




