Sequential Selective Dissolution of Coinage Metals in Recyclable Ionic Media
Abstract
Coinage metals Cu, Ag, and Au are essential for modern electronics and their recycling from waste materials is becoming increasingly important to guarantee the security of their supply. Designing new sustainable and selective procedures that would substitute currently used processes is crucial. Here, we describe an unprecedented approach for the sequential dissolution of single metals from Cu, Ag, and Au mixtures using biomass-derived ionic solvents and green oxidants. First, Cu can be selectively dissolved in the presence of Ag and Au with a choline chloride/urea/H2O2 mixture, followed by the dissolution of Ag in lactic acid/H2O2. Finally, the metallic Au, which is not soluble in either solution above, is dissolved in choline chloride/urea/Oxone. Subsequently, the metals were simply and quantitatively recovered from dissolutions, and the solvents were recycled and reused. The applicability of the developed approach was demonstrated by recovering metals from electronic waste substrates such as printed circuit boards, gold fingers, and solar panels. The dissolution reactions and selectivity were explored with different analytical techniques and DFT calculations. We anticipate our approach will pave a new way for the contemporary and sustainable recycling of multi-metal waste substrates.
Introduction
Coinage metals, namely Cu, Ag, and Au, have been heavily utilized throughout history and in modern times they prevalently serve as components of industrial and personal electronics. As their natural resources are limited, recycling from industrial and urban waste streams (including waste electrical and electronic equipment, WEEE) will support their availability. As secondary sources such as waste printed circuit boards (WPCBs) or waste solar panels have a higher metal content than average ores, recycling them is not only a necessity but also an economical opportunity.1 Yet, currently, dissolution processes for recycling metals are burdened with various issues regarding selectivity and environmental impact. Hence, the design of new sustainable methods is therefore necessary to ensure the security of noble metal supply in the future.2-11
Established recycling methods for noble metals include energy-consumptive pyrometallurgical roasting and smelting that produce large amounts of CO2 and other volatile pollutants (Figure 1). Traditional industrially applied methods for hydro- and solvometallurgical processing incorporate highly corrosive and dangerous mineral acids including aqua regia (HNO3/HCl) and piranha solution (H2SO4/H2O2), toxic lixiviants like cyanide (cyanide leaching), noxious oxidants like chlorine gas (hydrometallurgical chlorination) or even elemental mercury (amalgamation) simultaneously producing large amounts of wastewaters.12-14 Thiourea,15 thiosulphate,16 glycine,17 or iodine/iodide18 leaching, and others19-23 present significant progress regarding sustainability, but these methods, except in rare cases,24 suffer from various drawbacks, the most prevalent being poor selectivity.25-27 All metals dissolve under harsh and non-discriminatory conditions, which increases the number of total processing steps to acquire single, high-purity metals from complex WEEE substrates.
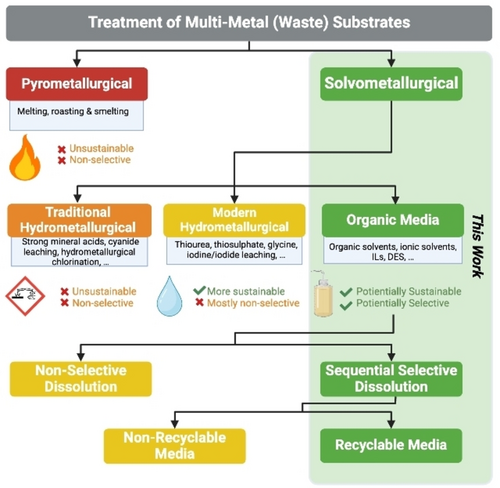
Different approaches to recycling of multi-metal waste substrates. This work focuses on solvometallurgical treatment of coinage metals in ionic organic media with an emphasis on sustainability, sequential selective dissolution, and recyclability (created with BioRender.com).
Utilization of (biomass-based) organic media for noble metal recycling28-38 seems to offer a paradigm shift towards selective metal dissolution with enhanced sustainability (Figure 1). Recent examples of single noble metal selective dissolution include the use of specific ligands39-41 or conditions.42-46 Selectivity can also be controlled by the type of oxidant47 or its concentration48 or is relying on dissolution kinetics.49 Deep eutectic solvents (DES),50, 51 a type of green ionic media, compared to ionic liquids (IL), present an affordable, safe, and potentially recyclable alternative with favorable features like low volatility and possible biodegradability. IL42, 52-60 and DES43, 44, 61-66 have been employed before in ionometallurgy to chemically dissolve noble metals using oxidants like Cu2+, Fe3+, I2, and HNO3. Given the diversity of biomass-derived ionic solvents—including organic acids67—DES hold an unexploited potential to become a next generation of sustainable media for noble metal dissolution and sequential treatment with inherent opportunity for selectivity.
Herein we present a selective, sequential dissolution of single metals from Cu, Ag, and Au mixtures by altering the combination of biomass-sourced ionic solvent and oxidant type. As shown here, metal interactions with ionic solvents and differences in oxidation potentials between Group 11 metals induce selectivity in the primary dissolution stage. This is the basis for straightforward, selective sequential dissolution of multi-metal substrates which is clearly advantageous over the conventional multi-step treatments. In addition, the ionic solvents were successfully recycled and reused as dissolution media (Figure 1). Our approach was trialed on real-life WEEE substrates, from which we achieved separation and selective retrieval of all three metals.
Results and Discussion
Choice of Solvent
Dissolution of noble metals in organic solvents is a concept of high interest and exhibits inherent opportunity for improved selectivity. The choice of a solvent is paramount when evaluating the dissolution procedures. Biomass-derived solvents are often advantageous, compared to ones from petroleum-based sources. This includes properties, such as low toxicity, high biodegradability, lower VOCs release, and reduced amount of (less or non-toxic) pollutants that are generated during their manufacture. Consequently, processes based on biomass-based solvents, in many cases, have advantages such as reduced disposal costs and improved worker safety. The utilization of biomass-based solvents is, therefore, expected to lower the environmental impact and play an important role in enhancing the sustainability of the processes.50, 51, 67, 68
Chloride anions can coordinate to the surface of an elemental metal like Cu and Au, thus reducing their oxidation potential and after oxidation stabilize the metal cations in the solution.2, 16, 25, 27 Following the reasoning above, we focused herein on inexpensive, biodegradable, and easy-to-prepare DES made of toxicologically well-characterized components, choline chloride (ChCl), a bulk chemical and additive for chicken food and naturally occurring urea (U).69, 70 This combination (reline) offers both high ionic strength as well as high chloride concentrations.50, 51 The DES was prepared beforehand by mixing 1 equivalent of ChCl and 2 equivalents of U at 80 °C to afford liquid, which remains in the liquid state even at room temperature.69, 70 For the following dissolution experiments metal wires were chosen as substrates. They were submerged into different dissolution media and weighed after specific times to quantify the amount of dissolved metal.
Dissolution of Cu
Using H2O2 (30 % aq.) as oxidant, Cu dissolves slowly in ChCl/U DES at 60 °C, and wire mass loss is plotted as a function of time in Figure 2. After 48 h the medium, consisting of 3714 mg of DES and 0.5 mL H2O2 (30 % aq), absorbs 2.7 wt % of Cu with an initial dissolution rate of 0.48 mol/m2h (See SI, section 2.). With increasing concentrations of dissolved Cu, the medium turns from green to blue, and finally, a blue precipitate of Cu2Cl(OH)3 starts to form (confirmed by EDS and XRD, Figures S2–S3). This sets the upper Cu concentration (3 wt %), where the system can work. It is, however, possible to modify the system to absorb any desirable amount of Cu since the amount of dissolved metal depends on the quantity of solvent and oxidant as well as other reaction parameters. Overall, the dissolution of Cu under benign conditions using green oxidant and safe DES gives clear advantages over treatment in noxious mineral acids like HNO3.
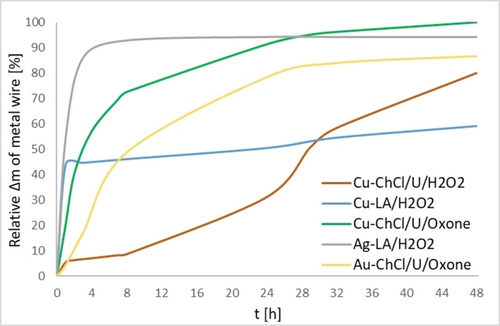
Relative Cu, Ag, and Au dissolution curves in different media are presented as % of wire mass loss (Δm) plotted against time. 145.6–135.0 mg of Cu wire with a diameter of 0.25 mm, 317.2 mg of Ag wire with a diameter of 0.25 nm, and 51.8 mg of Au wire with a diameter of 0.1 mm were submerged into 3714 mg of ChCl/U or LA combined with 0.5 mL of 30 % aqueous H2O2 or 0.5 g of Oxone at 60 °C for 48 h. Wires were weighted at specific times. The ChCl/U/H2O2 method is selective for Cu, the LA/H2O2 method is selective for Cu and Ag and the ChCl/U/Oxone method is selective for Cu and Au. The plateaus appear due to the loss of the oxidant (see Table S1 and Figure S1).
Selectivity
While the ChCl/U/H2O2 medium was successful in dissolving Cu, it was unable to etch either Ag or Au. The computational assessment of thermodynamics and plausibility of dissolution in ChCl/U for all three metals was conducted. This assessment was based on a thermodynamic cycle parametrized using DFT calculations with a hybrid cluster/continuum solvent model (Figure 3) employing the ωB97X-D489 functional and def2-TZVPPD91, 92 basis set as implemented in Orca. The thermodynamic cycle computes the free energy for oxidative dissolution of the metal in ChCl/U, by separating the chemical process into the free energy of dissolution in water and the solubility free energy when the metal ions are transferred from water to ChCl/U (For more information on the computational approach73-87 and details,88-105 see Supporting Information, section 5.). The computational results show that ChCl/U significantly stabilizes Cu2+, Ag+, and Au3+ cations and that oxidative dissolution is exergonic for all three metals with values of −78.4, −28.6, and −44.3 kcal/mol, respectively. These values are calculated with respect to the reduction of H2O2 in water. This finding suggests that oxidative dissolution of Ag and Au in ChCl/U should be thermodynamically possible by tuning the reaction conditions. Oxidation of the Ag wire surface was in fact observed and EDS measurements (Figure S9) confirmed the formation of an AgCl passivation layer—a more stable phase that was excluded from DFT calculations. Metallic gold remains in the ChCl/U solution, even in the presence of I2 or KBr,28 without mass loss or visible changes. A different oxidant is needed to dissolve Au.
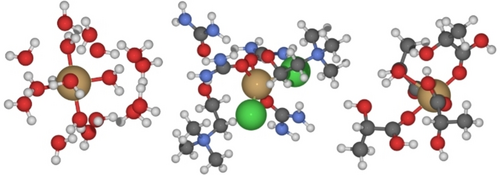
Optimized cluster structures using a cluster–continuum solvation method with ωB97X-D4 functional and the def2-TZVPPD basis set for Cu2+ ion in water (left), in ChCl/U (center), and in LA (right); atoms and ions are presented as spheres: Cu in brown, O in red, H in white, C in black, N in blue and Cl in green.
Dissolution of Au
The changing of H2O2 to Oxone, a sustainable inorganic oxidant, dissolution of both Cu and Au takes place in ChCl/U (Figure 2), while for Ag, passivation and deterioration of the wire surface was observed. The ChCl/U/Oxone suspension (3714 mg of DES and 0.5 g of Oxone) at 60 °C was able to dissolve 1.1 wt % of Au with an initial dissolution rate of 0.11 mol/m2h in the form of Au3+ as proven by UV/Vis spectroscopy and comparison to a solution of KAuCl4 (Figure S6). The formation of Cl2 was detected with the DPD indicator, but UV/Vis spectroscopy studies did not confirm its presence. However, we cannot exclude chlorine as a low-concentration, transient oxidant that is immediately consumed by metals in ChCl/U/Oxone. In the less reactive ChCl/U/H2O2 system, chlorine, if generated, likely reacts with the water present. This leaves peroxide as an active oxidant in this system and raises the desired selectivity in the dissolution (See SI, section 6.). As a result, the robust ChCl/U/Oxone system based on an inexpensive and green oxidant provides efficient dissolution of Au under mild reaction conditions. Importantly, the developed method has a clear advantage over the utilization of dangerous acids like aqua regia, expensive processes like iodine-iodide leaching, or dissolutions in various other organic solvents where large amounts of (expensive) organic ligands must be used.
Dissolution of Ag
Since Cl− passivates an Ag surface, a method for Ag dissolution should be based on non-chloride media. Different DES based on non-toxic and non-volatile weak acid that is produced naturally by fermentation, lactic acid (LA), and compounds containing amino groups like alanine, glycine, and NH4OAc were explored.67, 71, 72 It was finally realized that pure LA could dissolve Ag without any additives using just 30 % aqueous H2O2 as an oxidant. What is more, a high amount of dissolved Ag, 7.0 wt % was reached in this system (3714 mg of LA and 0.5 mL of 30 % aq. H2O2) after 24 h at 60 °C (Figure 2) with a high initial dissolution rate of 3.32 mol/m2h. H2O2 was in this case also a probable active oxidant species as the formation of peroxolactic acid was not observed by NMR (See SI, section 6.). Utilization of safe natural acids like LA for the dissolution of Ag opens a fascinating possibility for sustainable noble metal recycling.
Selectivity
DFT calculations were applied to determine the free energy of oxidative dissolution in LA, yielding values of −34.1, −9.2, and 49.5 kcal/mol for Cu2+, Ag+, and Au3+ ions, respectively, with respect to the reduction of H2O2 in water (Figure 3, right). The large positive value for Au implies that it is least likely to dissolve in LA, whereas the negative values for Cu and Ag suggest that the dissolution in the real systems might be thermodynamically allowed. In fact, no dissolution of Au was experimentally observed in LA/H2O2, while Cu dissolved readily, but reached only around a quarter of the amount of dissolved Ag in a given system which highlights the importance of specific metal-ion interactions.
Coinage Metals in Recyclable Ionic Media
When retrieving the dissolved metals from the solution and recycling of the dissolution media, metal species can be precipitated in antisolvents that would dissolve the ionic media.106 Here, we studied various antisolvents for the ChCl/U/H2O2 system containing copper and found that EtOH dissolves DES and precipitates Cu2+ as Cu2Cl(OH)3. It was later recognized that (NH4)2CO3 can be added to retrieve Cu2+ quantitatively as a mixed Cu salt (Figure 4, Figures S12–S13). In the next step, EtOH from the filtrate was removed, and the remaining ChCl/U was dried and recycled in 83 % in the average of three runs (See SI, section 3.1.). The observed slightly blueish color of regenerated DES indicated that traces of Cu2+ remain dissolved, yet not influencing the dissolution capability of reused DES. The possibility to close the recycling loop exhibits clear advantages over the treatment of metals in water-based media where precipitation with antisolvent is rarely viable.106
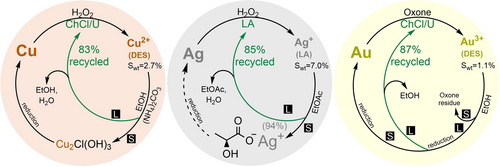
Closing the recycling loop for Cu-ChCl/U/H2O2 (left), Ag-LA/H2O2 (center) and Au-ChCl/U/Oxone (right) system. All percentages of recycled solvents and yield for Ag lactate are given as an average of three dissolution runs. Swt represents the solubility of the metal in the medium in wt % under reaction conditions: 145.6–135.0 mg of Cu wire with diameter of 0.25 mm, 317.2 mg of Ag wire with diameter of 0.25 nm and 51.8 mg of Au wire with diameter of 0.1 mm were submerged into 3714 mg of ChCl/U or LA combined with 0.5 mL of 30 % aqueous H2O2 or 0.5 g of Oxone at 60 °C for 48 h. S and L symbols indicate separation of solid and liquid phase, respectively.
Ag dissolved in LA/H2O2 can be treated with organic antisolvents like acetone or EtOAc to dissolve LA while Ag lactate is precipitated quantitatively as a gelatin-like material (Figure 4). Its identity was confirmed after drying by IR, UV/Vis, NMR, and EDS (Figures S16–S22) and comparing it to commercial Ag lactate. After the removal of water and EtOAc, LA was recovered at 85 % as an average of three runs and reused as dissolution media (See SI, section 3.2.). This example shows that benign organic media can be used to directly recover pure silver compounds and that provides a promising selective and straightforward approach for recycling of Ag in real applications.
When ChCl/U/Oxone system containing Au3+ was dissolved in EtOH, the precipitation of residual Oxone and its byproducts took place (Figure 4). As a showcase, the reduction of EtOH-soluble Au3+ ions could be achieved directly with Mg (Figure S23). The DES was recycled in 87 % on average of three runs and reused as dissolution media (See SI, section 3.3.). The treatment of the system with dissolved metal using antisolvents proved as an advantageous approach for closing the recycling loop by application of organo-soluble media for leaching.
Sequential Selective Dissolution of Coinage Metals
The results above provided us the opportunity to develop a sequential selective dissolution concept for coinage metals and multi-metal substrates like WEEE. The logical path for sequential treatment is to first dissolve Cu selectively in ChCl/U/H2O2 where Ag and Au are not soluble, then Ag in LA/H2O2, where Au is not soluble, followed by dissolving Au in ChCl/U/Oxone system (Table 1). It is worth mentioning that Ag starts to partially deteriorate (formation of AgCl, Figures S10–S11) after 32 h when Cu2+ ions are present in the ChCl/U/H2O2 system. This indicates that reaction times and ion concentrations are crucial parameters for optimizing selective dissolution steps for multi-metal substrates.
Sequential dissolution |
ChCl/U/H2O2 |
LA/H2O2 |
ChCl/U/Oxone |
|---|---|---|---|
Cu |
dissolution |
dissolution |
dissolution |
Ag |
passivation |
dissolution |
passivation |
Au |
no dissolution |
no dissolution |
dissolution |
Waste printed circuit boards (WPCBs) were selected as real-life WEEE substrates (Figure S25). First, 15 g of gold fingers with Au plating were soaked in DCM and acetone to remove silicones, brominated, and other possible coatings followed by aqueous HCl to dissolve the base metals that compose the solder. WPCB pieces were then treated twice in the ChCl/U/H2O2 system to dissolve all Cu, followed by the aforementioned recycling procedure. The precipitated Cu2Cl(OH)3 was cemented to obtain 111 mg of a powder where 98 % of the metal content was Cu (Table S6).
Due to the lack of Ag in the WPCB substrates, we focused directly on Au dissolution. The Au from detached connection plates (Figure S26) was subsequently leached in the ChCl/U/Oxone. After closing the recycling loop, 6 mg of a powder was obtained after cementation where 97 % of the metal content was Au demonstrating the feasibility of the applied approach in the recycling of real-life WEEE substrates (Table S7).
A different secondary source material was chosen to be leached in the LA/H2O2 system. Despite the prevalence of solar energy and the high silver consumption within this industry, recycling methods for Ag from solar panels are widely unexplored due to their complex composition. A waste solar panel consisting of a plastic motherboard, protection glass, ceramics, and Al and Ag electrodes held together with different adhesives and polymers was chosen as a suitable substrate.
We crushed and dismantled the selected solar panel by soaking the cut pieces in organic solvents and manually removing the adhesive layer between ceramics with Ag electrodes and protection glass (Figure S27). The Al electrode was first removed by soaking the crushed solar panel pieces (7 g) in 10 % aqueous HCl followed by extraction of Ag into the LA/H2O2 system. As the low silver concentration in LA prevents its precipitation as Ag lactate using EtOAc, Ag+ was precipitated as AgCl (35 mg) from an aqueous solution of NaCl where 99.9 % of metal content was Ag (Figure S28, Table S8), showing the potential of the system even for recycling solar panels.
The one metal – one method approach for sequential dissolution can also be applied when all three metals are present in WEEE. This was simulated by combining the WPCB square with Cu grid, Ag wire, and part of connection fingers with Au plating (Figure 5). After removing polymer coatings, plastics, and base metals, Cu, Ag, and Au were sequentially leached in ChCl/U/H2O2, LA/H2O2, and ChCl/U/Oxone systems, respectively. Due to the slow deterioration of Ag in the ChCl/U/H2O2 system when Cu2+ is present, it is essential to fine-tune the parameters like ion concentrations and leaching time when quantitative retrieval of metals is an objective.
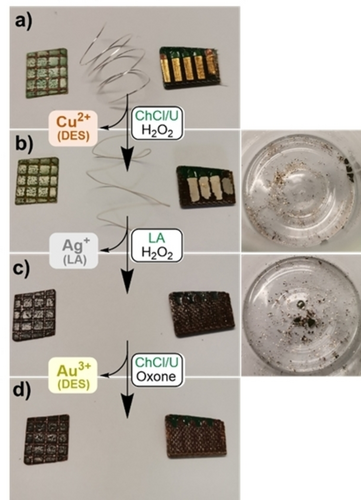
Applying the sequential dissolution to WPCB square with Cu grid, Ag wire, and piece of connection fingers with Au plating: a) before dissolution; b) after the dissolution of Cu in ChCl/U/H2O2 system – Cu is visibly gone, Ag wire starts to slowly deteriorate in the presence of Cu2+ and Au plating detaches from the connection fingers and appears as gold flakes at the bottom of the vial; c) after the dissolution Ag in LA/H2O2 system – Ag wire dissolves, but Au flakes remain in the vial; d) after the dissolution of Au in ChCl/U/Oxone system – Au flakes dissolve.
Comparative Analysis of the Methods
Compared to traditional recycling technologies that include dissolution in mineral acids and toxic lixiviants like cyanide as well as modern hydrometallurgical approaches like glycine, thiosulphate, cyanide, and iodine-iodide-based methods, the approaches presented herein are relatively inexpensive, environmentally sound, and moderately efficient (Table 2). An additional benefit of the current approach that helps to further reduce the overall environmental impact as well as cost is the recyclability of the dissolution media and the retrieval of pure metal species with a simple antisolvent approach. The methods presented herein exhibited lower starting dissolution rates (0.11–3.32 mol/m2 h) compared to dissolution in mineral acids and some other water-based methods including iodine-iodide leching (0.9 mol/m2 h),18 but were comparable to other methods using organic solvents, like leaching in organic aqua regia (0.3 mol/m2 h)5 or ligand-assisted leaching with I2 in acetone (0.18 mol/m2 h).36 However, the dissolution proceeded faster than in ligand-assisted dissolution in EtOH using O2 as an oxidant (0.06⋅10–3 mol/m2 h)38 and faster than in some traditional water-based methods like glycine leaching (0.001–0.04 mol/m2 h)17 and hydrometallurgical cyanidation (0.004 mol/m2 h).35 Additional exploration into life-cycle assessment is imperative to thoroughly examine the economic and environmental sustainability of the traditional and recent methods.
Metal |
Dissolution method |
Efficiency |
Cost |
Environmental impact |
|---|---|---|---|---|
Cu |
HNO3 |
high |
low |
high |
glycine leaching17 |
low |
low |
low |
|
this work: ChCl/U/H2O2 |
moderate |
low |
low |
|
Ag |
HNO3 |
high |
low |
high |
thiosulphate leaching16 |
high |
low |
low |
|
this work: LA/H2O2 |
moderate |
low |
low |
|
Au |
low |
low |
high |
|
iodine-iodide leaching18 |
high |
high |
moderate |
|
this work: ChCl/U/Oxone |
moderate |
low |
low |
Conclusion
In summary, we have developed procedures for sequential dissolution of Cu, Ag, and Au using non-volatile, recyclable, biomass-derived, and biodegradable ionic solvents prepared from toxicologically well-characterized components and green oxidants. Selectivity of dissolution and its origins were studied with different analytical techniques and supported by DFT calculations, which showed that the reactions can be understood and developed through basic coordination chemistry and thermodynamics. Pure metals or metal complexes can be retrieved along with recycling and reuse of the ionic media by simple treatment of metal dissolutions with antisolvents and presents a clear advantage over aqueous media procedures. With careful monitoring of the reaction parameters, our systems are applicable to real-life substrates such as waste printed circuit boards, gold fingers and waste solar panels without grinding pretreatment. With possible slight modifications, the developed methods are potentially applicable to different types of WEEE. The sustainable, sequential selective, and recyclable systems developed herein support the efforts of the circular economy. The adopted ionometallurgical strategy of utilizing mass-produced, inexpensive, and natural compounds, and green oxidants that are transformed to benign and/or recyclable waste to dissolve coinage metals with closed recycling loops fully aligns with green chemistry principles. The approaches developed in this work seem promising for scaling up because of overall moderate energy consumption and application of inexpensive and recyclable compounds.107, 108 However, to utilize the sequential dissolution in larger industrial applications further focus on parameters such as mass transfer during leaching and possible accumulation of species like Ni salts during recycling of the ionic media is needed. We anticipate that our novel approach will assist in guiding the development of contemporary methods for recycling of multi-metal waste substrates and support the security of noble metal supply.
Supporting Information
Detailed procedures, computational details, and spectra can be found in SI. The computational results (electronic and free energies as well as atomic structures) are available via IDA Fairdata service via link: https://doi.org/10.23729/796d99db-2969-4cd7-b622-11b3041cec42.
Acknowledgments
A. Z. acknowledges the Magnus Ehrnrooth Foundation for financial support. The authors acknowledge the financial support of the University of Helsinki and the use of ALD center Finland research infrastructure. The electronic structure calculations were made possible by computational resources provided by the CSC – IT Center for Science, Espoo, Finland (https://www.csc.fi/en/). T. W. and K. H. acknowledge Jane and Aatos Erkko Foundation for the funding for the LACOR project. M. M. M. acknowledges the Research Council of Finland (project 338228). The authors would like to thank Dr. Petra Vasko for her insightful comments on the manuscript.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




