Boosting the Electroreduction of CO2 to CO by Ligand Engineering of Gold Nanoclusters
Abstract
The electrochemical CO2 reduction reaction (CO2RR) has been widely studied as a promising means to convert anthropogenic CO2 into valuable chemicals and fuels. In this process, the alkali metal ions present in the electrolyte are known to significantly influence the CO2RR activity and selectivity. In this study, we report a strategy for preparing efficient electrocatalysts by introducing a cation-relaying ligand, namely 6-mercaptohexanoic acid (MHA), into atom-precise Au25 nanoclusters (NCs). The CO2RR activity of the synthesized Au25(MHA)18 NCs was compared with that of Au25(HT)18 NCs (HT=1-hexanethiolate). While both NCs selectively produced CO over H2, the CO2-to-CO conversion activity of the Au25(MHA)18 NCs was significantly higher than that of the Au25(HT)18 NCs when the catholyte pH was higher than the pKa of MHA, demonstrating the cation-relaying effect of the anionic terminal group. Mechanistic investigations into the CO2RR occurring on the Au25 NCs in the presence of different catholyte cations and concentrations revealed that the CO2-to-CO conversion activities of these Au25 NCs increased in the order Li+<Na+<K+<Cs+, and are gated by the cation-coupled electron transfer step. These results were confirmed by the Nernstian shifts of the polarization curves at different cation concentrations.
Introduction
The electrochemical carbon dioxide reduction reaction (CO2RR) has gained significant attention as a promising means to convert CO2 into value-added chemicals and fuels, and thereby to store excess renewable electricity.1 Among the products produced from the electrochemical CO2RR, CO holds special economic appeal because of its favorable market price and extensive uses in many industrially applied processes, such as carbonylation reactions and the Fischer–Tropsch synthesis.2 To date, various Au- and Ag-based nanomaterials have been developed as selective CO2RR catalysts for the production of CO.3 However, these materials are not well-defined at the atomic level, and generally exhibit polydisperse sizes, shapes, and surface structures, which often obscures their structure-property relationships.3a
Over the last decade, atomically precise metal nanoclusters (NCs) have emerged as a promising frontier in the electrochemical CO2RR.4 They can be synthesized in molecularly pure forms, and their structures can be crystallographically determined at the atomic level, which enables the detailed study of their catalytic structure-property relationships.5 Since the first report by Kauffman et al. in 2012,6 numerous Au- and alloy-based NCs have been developed as CO2RR electrocatalysts by tailoring their structures and compositions.7 In addition, Ag- and Cu-based NCs have been explored as selective electrocatalysts for producing CO and HCOO−, respectively.8 Notably, metal NCs have enabled the atomic-level identification of the active sites for this process. Using Au25(SR)18, Au38(SR)24, and Au144(SR)60 NCs (SR=thiolate) as model catalysts, it was previously revealed that the NCs undergo electrochemical activation by losing some ligands, and the dethiolated Au sites act as the active sites.7a It was also demonstrated that the highly active Au active sites can be transplanted into catalytically less-active Ag25(SR)18 and inactive Ni4(SR)8 NCs for efficient CO2-to-CO electroreduction.9
There has also been growing recognition that the local environments surrounding the active sites have a profound effect on the activity and selectivity of the CO2RR of a catalyst.10 In particular, alkali metals are known to play crucial roles in the CO2RRs taking place on gold, silver, and copper electrodes. More specifically, the CO2RR activities have been found to be critically dependent on the identity of the alkali metal, increasing in the order Li+<Na+<K+<Cs+.11 The origin of the cation effect has been initially explained by the Frumkin-type12 local field effect induced by the specifically adsorbed cations in the electric double layer.11c Recent studies have suggested that weakly hydrated cations are more concentrated at the outer Helmholtz plane (OHP) via non-covalent interactions, rather than undergoing specific adsorption, to give rise to high electric fields, which in turn would stabilize the reaction intermediates via adsorbate dipole-field interactions.13 In another theory developed by Chen et al.14 and Resasco et al.,15 local electrostatic interactions between the alkali metal cations and the CO2RR intermediates in the electric double layer were suggested to be the origin of the cation effect. This theory was further confirmed by Koper et al., who showed that in addition to the medium-range electric field-adsorbate dipole interactions, explicit short-range local electrostatic interactions also exist between the partially desolvated metal cations and the negatively charged reaction intermediates.16 Other theories have also been proposed to explain the cation effect on the CO2RR, such as the buffering effect of the cations at the interface, and the cation-dependent interfacial water structure.17
It has also been demonstrated that the local environment surrounding the catalytic site can be modified by introducing ionomers on the catalyst surface.18 Indeed, various ionomers have been adopted to modify the local concentrations of the cations, CO2, and H2O, in addition to the pH, through variation of their backbone chains and the charged moieties present at the ends of the side chains.19 However, the impact of ionomers on the CO2RR is still poorly understood, despite notable progress in recent years.20 Moreover, ionomers are known to be heterogeneous at the molecular level, and thereby pose significant challenges (e.g., catalyst segregation, active stie blockage, and a reduced electrode conductivity) when developing an optimized local environment.21 It is therefore important to design an electrocatalyst that contains highly active catalytic sites surrounded by tailored local environments at the molecular level.
Herein, we report a novel strategy to engineer the local environment of a Au25 electrocatalyst by introducing a cation-relaying ligand, namely 6-mercaptohexanoic acid (MHA). Au25(MHA)18 NCs (MHA-Au25) bearing carboxylate groups at the ends of the ligands are synthesized, and the resulting CO2-to-CO conversion activity is compared with that achieved by Au25(HT)18 NCs (HT-Au25; HT=1-hexanethiolate). Comparison of CO production on these Au25 NCs reveals that the CO2RR activity of the MHA-Au25 is significantly higher than that of the HT-Au25 when the catholyte pH is higher than the pKa of MHA, demonstrating the cation-relaying effect of the anionic terminal group. Mechanistic investigations of the CO2RR taking place on the Au25 NCs are carried out in the presence of different cations (Li+, Na+, K+, and Cs+) and catholyte concentrations to reveal the origin of the cation effects.
Results and Discussion
Ligand-protected metal NCs offer special advantages in that the metal core and ligand shell can be independently tailored to enhance their CO2RR activities.9, 22 Scheme 1 illustrates a strategy for boosting the CO2RR activity of Au25 NCs by employing cation-relaying ligands. As indicated, the Au25 NC structure is sufficiently open to allow small molecules (e.g., CO2 and H2O) to freely access the catalytic site, even in the presence of protecting ligands.7a In addition, the introduction of terminal anionic groups attracts cations, and relays them to the catalytic site, leading to an increased cation concentration close to the active site.
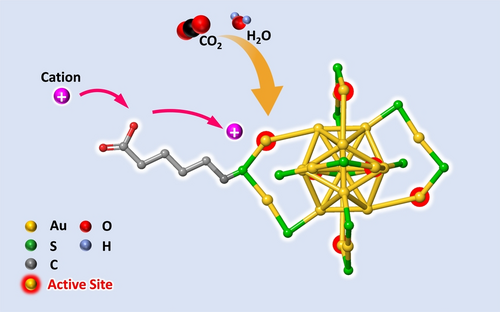
Schematic illustration of electrocatalytic CO2 reduction reaction taking place on a Au25 NC protected by cation-relaying ligands. Only one ligand is shown for clarity.
To investigate the effect of the cation-relaying ligand, the CO2RR activities of Au25 NCs protected with neutral (HT) and anionic (MHA) ligands were compared. The HT-Au25 and MHA-Au25 NCs were synthesized according to the literature.7a, 23 Both ligands are similar in length, but only the latter contains a terminal carboxylate group. As can be seen in Figure 1a, both Au25 NCs display distinct absorption peaks centered at 1.8 and 2.7 eV, which are characteristic absorption features of Au25 NCs.7a, 23 These similarities suggest that both Au25 NCs have comparable electronic structures despite being protected by distinctly different ligands. The synthesized Au25 NCs were further characterized by electrospray ionization (ESI) mass spectrometry. As shown in Figure 1b, the HT-Au25 NCs show a single peak at m/z 7034 Da, which is assignable to the [Au25(SC6H13)18]− ion. In contrast, the MHA-Au25 NCs exhibit three intense peaks with several minor peaks spaced evenly between them in the m/z range of 1500–4000 Da. These peaks correspond to MHA-Au25 NCs of different charge states and bearing Na+ counterions (see Figure S1). The experimental isotope pattern for the most intense peak at m/z ~2524 Da was superimposed with the simulated pattern [Au25(SC5H10COOH)18−2H+]3− (Figure 1b, inset), and this comparison confirmed the successful synthesis of the MHA-protected Au25 NCs. The combined absorption and mass analyses firmly established that the synthesized Au25 NCs are monodisperse with molecular purity.
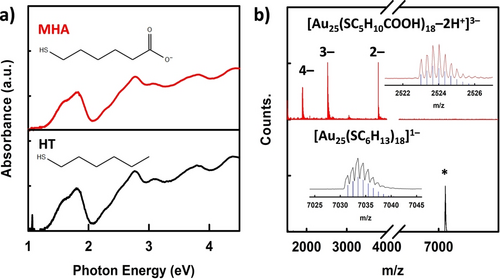
(a) Ultraviolet-visible absorption and (b) ESI mass spectra of the HT-Au25 NCs (black) and the MHA-Au25 NCs (red). The insets in part (b) show comparisons between the experimental data (lines) and the simulated isotope patterns (blue bars).
The CO2RR activities of the HT-Au25 and MHA-Au25 NCs were subsequently evaluated in a CO2-fed flow electrolyzer using 1.0 M KCl and 1.0 M KOH solutions as the catholyte and anolyte, respectively (Figure S2).7b The NCs were immobilized in a gas diffusion electrode (GDE), which was composed of a microporous layer (MPL) and a gas diffusion layer (GDL). Due to the extremely small sizes of the Au25 NCs (<3 nm), they were immobilized uniformly in the MPL (Figure S2b).7b Indeed, the hydrophobic interactions between the MPL and the NCs offered stable immobilization of the Au25 NCs, even for the water-soluble MHA-Au25 NCs.
It is well-known that the CO2RR activities of thiolate-protected Au NCs undergo electrochemical activation by losing some of their ligands under the application of a reduction potential.7a, 9 The undercoordinated Au sites exposed by ligand removal serve as active sites, leading to a significantly enhanced CO2RR activity. In a previous study, our group revealed that the HT-Au25 NCs were converted into highly active catalysts upon losing their thiolate ligands under reductive conditions;7a a similar electrochemical activation process was observed for the MHA-Au25 NCs prepared herein. As compared in Figure S3a, both the HT-Au25 and MHA-Au25 NCs exhibited a similar pattern of current increase and stabilization during constant potential electrolysis (CPE) at an applied potential of −1.79 V versus the standard hydrogen electrode (SHE; hereafter, all potentials are reported vs SHE unless noted otherwise). However, the current density of the MHA-Au25 NCs was stabilized at a significantly higher current density (~100 mA/cm2) than that of the HT-Au25 NCs (~80 mA/cm2).
Due to the strong interactions between the Au25 NCs and the GDE after CPE, it was extremely difficult to retrieve the activated Au25 NCs from the GDE for analysis. Therefore, elemental mapping analysis was conducted for the Au NCs immobilized on GDE using energy dispersive X-ray spectroscopy (EDS). This analysis was particularly useful for imaging the local environments surrounding the Au25 catalysts. Figures S4a and S4b show the cross-sectional scanning electron microscopy (SEM) and EDS mapping images of the Au25 NCs/GDE after activation at −1.79 V for 30 min in a 1.0 M KCl catholyte. The EDS mapping images recorded for Au and S indicate that the Au25 NCs were well dispersed in the GDE, being mainly immobilized in the MPL of the GDE. To further evaluate the compositions of the Au25 NCs after activation, the atomic ratios of Au and S were determined using the fluorine component of polytetrafluoroethylene, which was embedded into the GDE as an internal standard. As a result, the relative ratios of Au and S were determined to be 1.01 and 0.48 % (with respect to the internal standard) for HT-Au25 NCs and 0.83 and 0.36 % for MHA-Au25 NCs (Figure S4c). From these values, the chemical formulae of the activated HT-Au25 and MHA-Au25 NCs were defined as Au25(SC6H13)12.2 and Au25(SC5H10COOH)10.4, respectively (Figure S4d). These results clearly confirm that both Au25 NCs are electrochemically activated by partial ligand removal under reductive conditions, which exposed ~6 and ~8 de-ligated Au active sites, respectively. However, considering the atomic structure of the Au25 NCs, which possesses six staple motifs (Scheme 1),24 and the uncertainties associated with the EDS measurements, it is reasonable to assume that both NCs are activated by losing six ligands (one from each staple motif).7a The predicted structure of the activated Au25 NC is presented in Figure S4e. Additionally, transmission electron microscopy analyses of the HT-Au25/GDE and MHA-Au25/GDE showed that the NC core sizes were preserved at ~1.2 nm after CPE experiment at −1.79 V for 2 h (Figure S5). This result indicates that the Au25 NCs were still well-protected from aggregation even after losing some ligands. The linear sweep voltammetry (LSV) curves recorded before and after the electrochemical activation process show that the catalytic current densities for both NCs were significantly enhanced after electrochemical activation (Figure S3b). Interestingly, the current densities recorded for the activated MHA-Au25 NCs were significantly higher than those of the HT-Au25 NCs at the same applied potential.
To identify the active sites present in the activated Au25 NCs, operando attenuated total reflection (ATR) Fourier transform infrared (FT-IR) spectroscopy was employed25 during the CO2RR to capture the intermediates produced on the catalyst surface (see the Supporting Information for experimental details). Figure 2 displays the operando FT-IR spectra obtained from the HT-Au25 and MHA-Au25 NCs during the CPE measurements carried out in a CO2-saturated NaClO4 solution (1.0 M). When a potential of −1.79 V was applied, a band was observed at ~1940 cm−1 for both Au25 NCs, which corresponds to the stretching mode of the *CO species that are linearly adsorbed on the Au surface.26 This indicates that both NCs possess similar undercoordinated Au active sites, which are exposed by ligand loss after electrochemical activation. The high absorbance of the CO band observed for the MHA-Au25 NCs is consistent with the high CO2RR activity of this catalyst. The other bands observed at ~1620 and ~1390 cm−1 were assigned to the H2O bending mode and the asymmetric stretch mode of the bicarbonate species (HCO3−/CO32−),26b, 27 respectively, whose concentrations change at the catalyst-electrolyte interface during CO2 electrolysis.
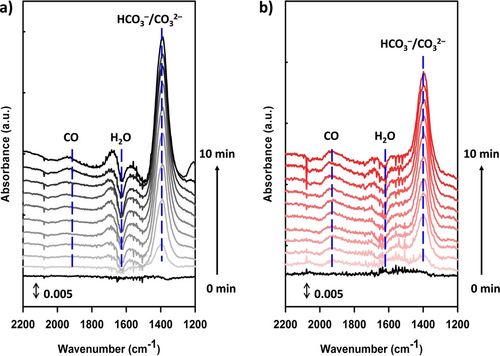
Operando ATR FT-IR spectra on the (a) HT-Au25/GDE and (b) MHA-Au25/GDE in CO2-saturated 1.0 M NaClO4 electrolyte solutions (pH 4.5) during CPE measurement at −1.79 V vs SHE. A NaClO4 electrolyte was used to monitor the (bi)carbonate species generated during the CO2RR.
Figure 3a shows the LSV traces recorded on the activated HT-Au25 and MHA-Au25 NCs in Ar-saturated (dashed line) and CO2-saturated (solid line) atmospheres, which represent the current densities for the hydrogen evolution reaction (HER) and the CO2RR, respectively. While the CO2RR activity of the MHA-Au25 NCs was significantly higher than that of the HT-Au25 NCs, their HER activities were comparable, indicating that the ligand effect was particularly significant for the CO2RR. The CO2RR activity and selectivity of the Au25 NCs were further compared by conducting CPE measurements at various applied potentials. As shown in Figure 3b, the partial current density of CO (jCO) generated from the HT-Au25 NCs increased upon decreasing the applied potential. The CO selectivity remained >95 % in the potential range of −0.9 to −1.3 V, before dropping to 87 % at −1.4 V. In contrast, the jCO values observed for the MHA-Au25 NCs were significantly higher than those of the HT-Au25 NCs, and the CO selectivity was >95 % at potentials higher than −1.2 V. For example, at −1.1 V, the jCO value of the MHA-Au25 NCs was 100 mA/cm2, which is more than double that of the HT-Au25 NC (i.e., 46 mA/cm2). However, the CO selectivity was sharply decreased to below 80 % at higher jCO value (>300 mA/cm2) due to the enhanced HER in the flooded MHA-Au25/GDE. In addition, the charge-transfer resistance (RCT) determined by electrochemical impedance spectroscopy (EIS) was significantly reduced for the MHA-Au25 NCs compared to the HT-Au25 NCs (Figure 3c), indicating that charge transfer from the NC catalyst to the CO2 molecules was greatly enhanced in the former.
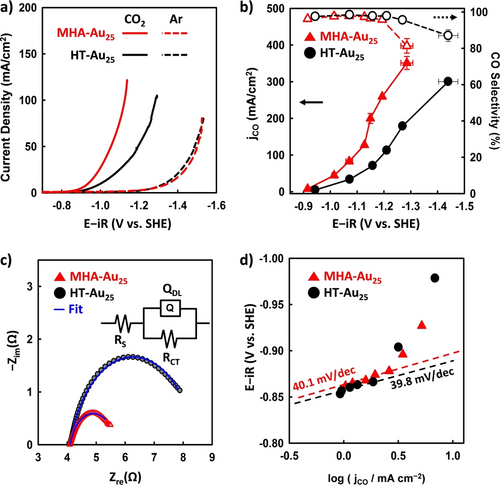
(a) LSV traces recorded at 3 mV/s on HT-Au25 and MHA-Au25 NCs in CO2-fed and Ar-fed electrolyzers. (b) jCO data acquired on the HT-Au25 and MHA-Au25 NCs and the corresponding CO selectivities as a function of the cathodic potential. (c) Nyquist plots measured at −0.99 V for the HT-Au25 and MHA-Au25 NCs. The equivalent circuit used to fit the EIS data is illustrated in the inset (RS, solution resistance; RCT, charge-transfer resistance; QDL, constant phase element for the double layer). (d) Tafel plots constructed for the electrochemical CO2RR on the HT-Au25 and MHA-Au25 NCs in a 1.0 M KCl solution. Error bars represent the standard deviation of three separate measurements. Potentials in panel a,b, and d were iR-corrected.
Tafel slopes of 120 and 40 mV/dec are expected when the reaction is gated by the first [Eq. (1)] and second electron transfer [Eq. (3)] steps, respectively.28, 29 The similarly low Tafel slopes observed for the two NCs suggest that the electroreduction of CO2 proceeds in a similar pathway, wherein the first electron transfer step is greatly facilitated, and the second electron transfer step is involved in the rate-determining step (RDS).7a
Since the CO2RR process can further be controlled by the proton transfer processes [Eqs. (2) and (4)],30 the effects of proton transfer on the CO2RRs of these two NC catalysts were investigated by varying the catholyte pH (1–14) while maintaining a constant K+ concentration (1.0 M). Figure 4a shows the jCO values obtained for the HT-Au25 NCs as a function of the applied potential at three different catholyte pH conditions. Interestingly, the jCO values were collapsed in SHE potential scale regardless of the catholyte pH, which indicates that the RDS is not associated with proton transfer. In addition, it was found that the CO selectivities were >95 % in the potential region from −0.9 to −1.3 V, and that they gradually decreased at higher overpotentials (Figure S6a). In contrast, the jCO values obtained for the MHA-Au25 NCs exhibited two different activity curves for pH ranges of 5–14 and 1–3 (Figure 4b). It should be noted here that the observed pH dependence of the jCO values is distinctly different from those expected for the proton-transfer-coupled CO2RR, which would increase with a decreasing pH.
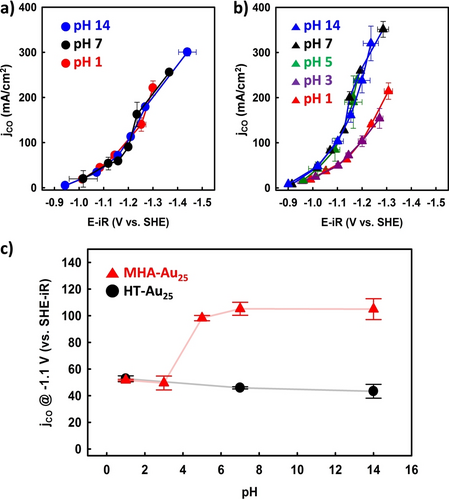
jCO data obtained for the (a) HT-Au25 and (b) MHA-Au25 NCs under different pH conditions. The cation (K+) concentration of catholyte was fixed at 1.0 M. (c) Comparison of the jCO values obtained for the HT-Au25 and MHA-Au25 NCs as a function of the catholyte pH. Error bars represent the standard deviation of three separate measurements. Potentials in panel a and b were iR-corrected.
Figure 4c compares the jCO values obtained at −1.1 V for the HT-Au25 and MHA-Au25 NCs at different pH conditions. Although the jCO values of the HT-Au25 NCs are comparable under all pH conditions, those of MHA-Au25 NCs are significantly higher at pH values ≥5. More specifically, jCO values were of ~50 mA/cm2 were recorded at pH 1 and 3, whereas values >100 mA/cm2 were observed at higher pH values. The CO selectivities were >90 % under all pH conditions evaluated herein (Figure S6b), indicating that the reduced jCO values observed at low pH conditions are not caused by the competing HER in acidic media.
It is well-documented that the CO2RR activity can be significantly boosted in the presence of alkali metal cations that effectively stabilize the CO2RR intermediate.13-16, 31 The sudden increase in the CO2RR activity of the MHA-Au25 NCs observed at pH values ≥5 can therefore be ascribed to the change in the cation-relaying MHA ligand. Considering the pKa value of MHA (3.7), the cation-relaying effect is expected at higher pH values (>pKa), wherein the dissociated carboxylate species effectively relay cations to the nearby catalytic sites. When the pH is below the pKa of MHA, the carboxylate moieties of the ligand are fully protonated, and the cation-relaying effect no longer occurs. To verify this hypothesis, ion-paired MHA-Au25 NCs were prepared by binding the carboxylate groups of MHA with n-tetraoctylammonium (TOA) cations, according to a previously reported procedure (see also Figure S7a).32 The TOA-bound MHA-Au25 NCs exhibited the characteristic absorption profile of the Au25 NCs, indicating that the NC structure was preserved (Figure S7b). As can be seen in Figure S8a, the TOA-bound MHA-Au25 NCs exhibited similar jCO values at pH 1, 7, and 14, displaying pH independent CO2RR activities. In addition, their CO2RR activities were similar to those of the HT-Au25 NCs (Figure 4a), thereby demonstrating that the enhanced CO2RR activity exhibited by the MHA-Au25 can be effectively blocked by ion-paring their carboxylate groups with TOA cations. This result confirms the cation-relaying effect of the MHA ligand under pH >pKa conditions, and further demonstrates that both the HT-Au25 and MHA-Au25 NCs contain the same number of active sites, confirming the assumption made for the activated Au25 NCs.
To further confirm the cation-relaying effect of the MHA ligand, SEM and EDS cross-sectional images of the HT-Au25/GDE and MHA-Au25/GDE were examined after conducting CPE experiments in a 1.0 M KCl solution at −0.99 V vs SHE for 30 min. To avoid interference from catholyte flooding into the GDE, CPE was conducted at a low overpotential for a short period of time. Figures 5a and 5b show cross-sectional SEM and EDS images of the two electrodes, in which the yellow dashed lines indicate the border of the MPL region. The EDS mapping images for Au show that both the HT-Au25 and MHA-Au25 NCs are predominantly immobilized in the hydrophobic MPL region. Surprisingly, the EDS mapping images for K show that the K+ ions are mainly accumulated in the MPL region of the MHA-Au25/GDE, whereas they are evenly distributed throughout the MPL and GDL regions of the HT-Au25/GDE. The relative atomic ratios of Au and K obtained from two electrodes are compared in Figure 5c. While the similar Au ratios indicate that the Au25 loadings were well-controlled to a similar level (0.63–0.74 %), the K+ ion content was remarkably higher in the MHA-Au25/GDE (1.59 %) than in the HT-Au25 (0.37 %). These data indicate that a cation-rich environment was created during CO2 electroreduction in the MHA-Au25/GDE, unambiguously confirming the cation-relaying effect of the MHA ligands. Furthermore, the atomic ratio of K+ is much higher than that of Cl− and the difference is significantly more pronounced for the MHA-Au25 NCs (Table S1), suggesting that the cation-rich environment was mainly created by the cation attraction effect, not by the catholyte flooding. After catalysis, more K+ ions appeared to be paired with the carboxylates of the MHA and/or the bicarbonate species (HCO3−/CO32−) generated during the CO2RR in the MHA-Au25/GDE.
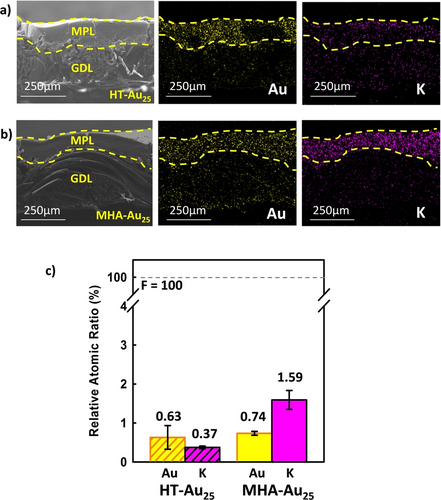
Cross-sectional SEM (left) and EDS mapping images of the Au (middle) and K (right) elements present in the (a) HT-Au25/GDE and (b) MHA-Au25/GDE after conducting CPE experiments in a 1.0 M KCl solution at −0.99 V vs SHE for 30 min. The yellow broken lines denote the MPL border. (c) Relative atomic ratios of Au and K obtained from EDS analyses of the HT-Au25/GDE and MHA-Au25/GDE. The atomic ratios were quantified based on the F component of the GDE as an internal standard. Error bars in panel c represent the standard deviation of quantitative analysis results measured at three different locations on the electrode.
To further probe the cation-relaying effect of this system, the jCO values obtained from CO2 electrolysis on the HT-Au25 and MHA-Au25 NCs at −1.1 V were compared at various KCl concentrations (0.1–3.0 M). Both Au25 NC catalysts exhibited increased jCO values at higher catholyte concentrations, indicating that the CO2RR activity is highly dependent on the interfacial cation concentration close to the active sites.33 Notably, the jCO values obtained for the MHA-Au25 NCs were more than double those obtained for the HT-Au25 NCs at all catholyte concentrations evaluated herein (Figure 6a). To further examine the CO2RRs of the HT-Au25 and MHA-Au25 NCs at different KCl concentrations, the jCO values were compared with respect to the SHE. As shown in Figure S9, the jCO values are considerably different on the SHE scale and increase with the KCl concentration. It should be noted that the potentials were iR corrected and thus the difference in jCO values was not caused by the different ion conductivities of the catholytes of different KCl concentrations. In a recent study, Shin et al. used experimental and simulation results to propose a cation-coupled mechanism as the RDS of the CO2-to-CO conversion carried out on a polycrystalline Ag electrode.31 They demonstrated the Nernstian shift of the polarization curves upon varying the cation concentration by plotting the polarization curves with respect to the cation concentration-corrected electrode (CCE) scale, defined as CCE=SHE−0.059×log[K+], where [K+] denotes a bulk cation concentration.31 Re-plotting the polarization curves in various KCl concentrations with respect to the CCE scale resulted in a single line for both the HT-Au25 and the MHA-Au25 NCs (Figure 6b and Figure S9). The fact that the polarization curves obtained at different KCl concentrations collapsed into a single line implies that the cation-coupled mechanism constitutes the RDS for the CO2-to-CO conversion in both Au25 NCs. Furthermore, the upward shift of the polarization curves obtained for the MHA-Au25 NCs suggests that the K+ concentration in the proximity of the MHA-Au25 NCs is significantly higher than that of the HT-Au25 NCs at the same bulk K+ concentration. This result is consistent with the EDS mapping images (Figures 5a–b). In previous studies on the CO2RR carried out over Au and Ag electrodes, the partially desolvated cations (e.g., K+ and Cs+) were identified as crucial promoters of the CO2 reduction process due to their role in stabilizing the *CO2− intermediate [Eq. (1)].16 In the present study, the Tafel results (Figure 3d) revealed the second electron transfer step as the RDS for the CO2-to-CO conversion [Eq. (3)] on both Au25 NCs. Thus, the cation concentration-dependent jCO values presented in Figure 6b suggest that the CO2-to-CO conversion process is gated by the cation-coupled second electron transfer step.

(a) jCO values measured at −1.1 V and (b) polarization curves measured in various catholyte solutions (0.1–3.0 M KCl) for the HT-Au25/GDE and MHA-Au25/GDE. In (b), the polarization curves are plotted on the CCE scale, where CCE=SHE − 0.059×log[K+]. (c) jCO and the corresponding CO selectivities measured for the HT-Au25/GDE and MHA-Au25/GDE at −1.1 V in different catholytes containing 1.0 M MCl (M=Li+, Na+, K+, and Cs+). (d) Relative atomic ratios of cations quantified from the EDS mapping images of the HT-Au25/GDE and MHA-Au25/GDE, as presented in Figures 5 and S10. Error bars in panel d represent the standard deviation of quantitative analysis results measured at three different locations on the electrode.
After observing the cation effect of K+, the variation in the CO2RR activity was investigated for different cations. Figure S10 shows the jCO and corresponding CO selectivities obtained for the HT-Au25 and MHA-Au25 NCs in catholytes containing 1.0 M LiCl, NaCl, KCl, or CsCl. As presented in the Figure, the jCO values increase as the cation size increases (i.e., Li+<Na+<K+<Cs+), although this effect is significantly more pronounced for the MHA-Au25 NCs. As can be seen in Figure 6c, the jCO values obtained for the MHA-Au25 NCs at −1.1 V are more than 2-fold and 3-fold higher than those obtained for the HT-Au25 NCs in the presence of K+ and Cs+, respectively. This result indicates that the cation effect is more significant for large cations, such as K+ and Cs+. In a previous study, the higher activity observed for the reduction of CO2 over copper in the presence of large cations was ascribed to a high concentration of such cations in the OHP.15 Recently, Koper et al. used experimental and theoretical investigations to propose that the cation effect arises both from the different surface concentrations at the OHP and the different abilities of these cations to coordinate to the reaction intermediate.16
Although the large cation effect was proposed to originate from the mixed effect of the higher concentration at the OHP and the coordinating ability of large cations,13-16, 31 the present study clearly decouples these two considerations. Figure 6d shows the relative atomic ratios of the different cations (M+), as quantified from the EDS mapping images of the HT-Au25/GDE and MHA-Au25/GDE (see Figures 5a, b, and S11). As shown in Figure 6d, the relative atomic ratios of Na+, K+, and Cs+ differ significantly between the HT-Au25/GDE and MHA-Au25/GDE, although comparable values were obtained for each cation identity (on average, 0.28 (±0.11) and 1.83 (±0.29), respectively). This result demonstrates that the cation effect observed in the Au25/GDE system originates from the cations themselves rather than their concentrations. In a previous density functional theory (DFT) study, Koper et al. revealed that large cations possess partially desolvated coordination shells and show stable coordination with CO2, which stabilizes the negatively charged reaction intermediates via local interactions.16 The large cation effects observed here can therefore be understood by considering the local electrostatic interactions between the cations and reaction intermediate (*COOH− in this work). Thus, large cations that exhibit superior binding abilities to the negatively charged reaction intermediate can stabilize the intermediate, ultimately promoting electron transfer from the Au active sites to the intermediate. Although the large cation effect is clearly observed for these Au25 NCs, it is greatly enhanced in the presence of MHA, a cation-relaying ligand, which attracts cations and delivers them close to the active sites.
Finally, the long-term stability of the MHA-Au25/GDE system was examined by conducting CPE at −1.79 V vs SHE in a CO2-fed flow electrolyzer with a flowing 1.0 M KCl electrolyte at pH 7. As presented in Figure 7, the MHA-Au25/GDE exhibited an excellent electrocatalytic stability, with a high jCO of ~100 mA/cm2 and a CO selectivity of >97 % over 24 h of operation. These results clearly indicate that the MHA-Au25 NCs exhibit a stable cation-relaying effect, which promotes a high CO2RR activity. Thus, the MHA-Au25 NCs constitute highly active yet stable CO2RR catalysts with an efficient cation-relaying function.
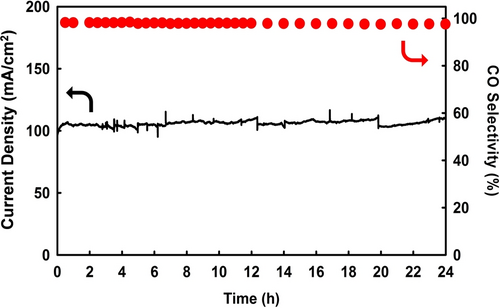
Long-term CO2-to-CO electrocatalysis activity of the MHA-Au25 NCs, as evaluated based on the current density and the CO selectivity at −1.79 V vs SHE in a 1.0 M KCl solution over 24 h.
Conclusion
A highly active CO2RR electrocatalyst was successfully prepared by the ligand engineering of Au25 NCs with a cation-relaying MHA ligand. Operando FT-IR analysis of the synthesized MHA-Au25 and HT-Au25 NCs during the CO2RR revealed that both NCs possess Au active sites that are exposed after ligand loss. These catalysts produced CO from CO2 with a >90 % selectivity. However, the CO2RR activity of the MHA-Au25 NCs was significantly higher than that of the HT-Au25 NCs when the catholyte pH was higher than the pKa of MHA, demonstrating the cation-relaying effect of the anionic terminal group. Mechanistic investigations carried out using different cations and different catholyte concentrations revealed that the CO2-to-CO conversion of the Au25 NCs was gated by the cation-coupled electron transfer step, which increases in the order Li+<Na+<K+<Cs+. The cation-coupled CO2RR process was unambiguously underpinned by the Nernstian shifts of the polarization curves upon variation in the cation concentration. Elemental mapping analysis of the local environments surrounding the Au25 NCs further revealed that the local cation concentrations in the proximity of the MHA-Au25 NCs were significantly higher than those in the proximity of the HT-Au25 NCs, although similar values were obtained regardless of the cation identity. It was therefore concluded that the cation effect imparted by the large cations originates from their high binding ability to the reaction intermediate. This study opens new routes to the atomic-level engineering of the local environment as an effective means to boost the CO2RR activities of metal NCs.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grants (no. NRF-2022R1A2C3003610) and the Carbon-to-X Project (Project no. 2020M3H7A1096388) through the NRF funded by the Ministry of Science and ICT, Republic of Korea. This work was supported in part by the Yonsei University Research Fund (Post Doc. Researcher Supporting Program) of 2024 (project no.: 2024-12-0026).
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




