Syntheses and Structures of 5-Membered Heterocycles Featuring 1,2-Diphospha-1,3-Butadiene and Its Radical Anion
Abstract
LGa(P2OC)cAAC 2 features a 1,2-diphospha-1,3-butadiene unit with a delocalized π-type HOMO and a π*-type LUMO according to DFT calculations. [LGa(P2OC)cAAC][K(DB-18-c-6)] 3[K(DB-18-c-6] containing the 1,2-diphospha-1,3-butadiene radical anion 3⋅− was isolated from the reaction of 2 with KC8 and dibenzo-18-crown-6. 3 reacted with [Fc][B(C6F5)4] (Fc=ferrocenium) to 2 and with TEMPO to [L−HGa(P2OC)cAAC][K(DB-18-c-6)] 4[K(DB-18-c-6] containing the 1,2-diphospha-1,3-butadiene anion 4−. The solid state structures of 2, 3K(DB-18-c-6], and 4[K(DB-18-c-6] were determined by single crystal X-ray diffraction (sc-XRD).
Introduction
Stable phosphorus-centered radicals have attracted wide interest due to their intriguing electronic structure, bonding situation, reactivity, and photophysical properties,1 and because they are intermediates in chemical reactions.2 Neutral,3 cationic,4 and anionic5 phosphorus-centered radicals have been kinetically stabilized by the use of bulky ligands and/or N-heterocyclic carbenes (NHCs) and cyclic (alkyl)(amino)-carbenes (cAACs), and by π-delocalization of the unpaired electron spin density.1 However, phosphorus-containing radicals of π-conjugated systems are scarce despite the ease with which phosphorus can be incorporated into such systems.
1,3-Butadiene is the simplest organic molecule with conjugated π bonds, and P-containing 1,3-butadienes are also known.6 However, while one-electron oxidation and reduction of 1,3-butadiene gave the radical cation and anion,7 only radical cations of P-containing butadienes have been reported, whereas P-containing 1,3-butadiene radical anions are unknown, to date. The 2,3-diphospha-1,3-butadiene radical cations I⋅+ and II⋅+ (Figure 1) and the 2-phospha-3-aza-1,3-butadiene radical cation III⋅+ were synthesized by Bertrand et al.,8, 9 while Ghadwal et al. prepared the 2-phospha-1,3-butadiene radical cations IV⋅+.10
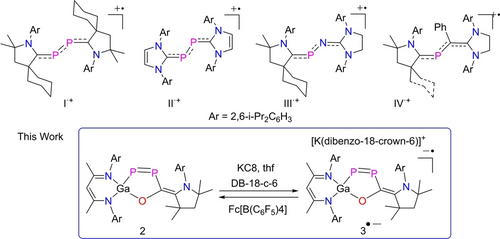
Phosphorous containing 1,3-butadiene radical cations I⋅+-IV⋅+ and 1,2-diphospha-1,3-butadiene radical anion 3⋅− (this work).
We recently showed that electropositive L(X)Ga substituents stabilize open-shell and π-bonded pnictogen compounds11 and other unusual bonding situations.12 Moreover, gallaphosphenes13 were found to undergo cycloaddition and E−H (E = N, O, S, Se) bond activation reactions,13, 14 and carbene-coordinated diphosphenes were oxidized to radical cations.15 We report here the synthesis of LGa(P2OC)cAAC 2 (L = HC[C(Me)N(Ar)]2; Ar = 2,6-i-Pr2C6H3) with a 1,2-diphospha-1,3-butadiene moiety, its radical anion 3⋅− and the 1,2-diphospha-1,3-butadiene anion 4−.
Results and Discussion
LGa(P2OC)cAAC 2 was synthesized by reaction of LGa(PCO)2 113b with cAAC and isolated as a violet microcrystalline powder (Scheme 1). 2 dissolves in toluene and THF and is stable in both solution and solid state for weeks under an inert gas atmosphere at ambient temperature, whereas decomposition occurs upon exposure to air and moisture.
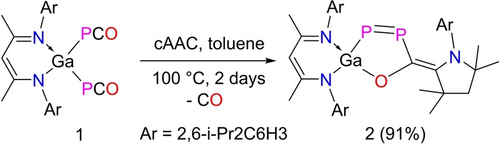
Synthesis of 1,2-diphospha-1,3-butadiene 2.
The 1H and 13C NMR spectra of 2 show two sets of resonances for the Ar groups of the β-diketiminate and cAAC ligands. The 31P{1H} NMR spectrum shows two doublets at 484.1 (d, 1JPP=583 Hz) and 144.6 ppm (d, 1JPP=583 Hz) for the magnetically inequivalent P atoms, which are shifted to lower field compared to other phosphabutadienes (+52 to +268 ppm),8-10 but compare well with asymmetric metalladiphosphenes.1d, 16
The molecular structure of 2 in the solid state was determined by sc-XRD (Figure 2). 2 crystallizes in the monoclinic space group P21/n with one independent molecule and disordered solvent molecule (n-hexane) in the asymmetric unit.17 The short P1−P2 (2.0662(5) Å) and C30−C31 (1.399(2) Å) bonds within the planar P2C2 π-conjugated unit are comparable to other P−P and C−C double bonds1d, 1e, 8, 15 and calculated values.18a The P2−C30 (1.7919(14) Å) bond length is also consistent with the sum of the calculated single bond radii (P 1.11 Å; C 0.75 Å),18b and the Ga1−P1 (2.2844(4) Å) and C31−N3 (1.3815(18) Å) bond lengths of 2 agree with experimental Ga−P14, 15 and C−N1, 8-10 single bonds, respectively. The five-membered GaP2OC heterocycle is coplanar with the P2C2 π-conjugated system, and the C3N2Ga metallacycle is perpendicular to this plane.
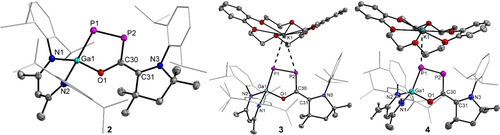
Molecular structures of compounds 2, 3[K(DB-18-c-6)], and 4[K(DB-18-c-6)]. Thermal ellipsoids set at 50 % probability; Hydrogen atoms (except CH2 in 4[K(DB-18-c-6)]), solvent molecules and alternate positions of disordered parts omitted for clarity. Selected bond lengths (Å): 2: Ga(1)−O(1) 1.8738(10), Ga(1)−P(1) 2.2844(4), P(1)−P(2) 2.0662(5), P(2)−C(30), 1.7919(14), O(1)−C(30) 1.3687(16), C(30)−C(31) 1.399(2), N(3)−C(31) 1.3815(18); 3[K(DB-18-c-6)]: Ga(1)−O(1) 1.8633(7), Ga(1)−P(1) 2.2418(3), P(1)−P(2) 2.1620(4), P(2)−C(30) 1.8202(9), O(1)−C(30) 1.3756(11), C(30)−C(31) 1.3805(13), N(3)−C(31) 1.4259(12), P(1)⋅⋅⋅K(1) 3.2426(4), P(2)⋅⋅⋅K(1) 3.5965(4); 4[K(DB-18-c-6)]: Ga(1)−O(1) 1.8863(15), Ga(1)−P(1) 2.3068(6), P(1)−P(2) 2.0653(7), P(2)−C(30) 1.787(2), C(30)−C(31) 1.394(3), N(3)−C(31) 1.373(3), O(1)−C(30) 1.354(3), P(1)−K1 3.421(4).
Density functional theory (DFT) calculations at the PBE0-D3BJ/def2-TZVP level of theory provided insight into the electronic structure of 2. The DFT-optimized geometry of 2 agrees well with the solid-state structure (Figure S23). The different electronegativities of Ga and C result in a charge separation between the P atoms, so that the P2 atom (+0.29 e) has a higher positive NBO charge than the P1 atom (−0.53 e). The localization at the P atom amounts to 68 % for the Ga1−P1 NBO and only 36 % for the P2−C30 NBO. The calculations further revealed that the HOMO of 2 (−4.68 eV) corresponds to a π-type orbital which is delocalized over the P2C2N unit with a small contribution from the pz-type orbital of the O atom (Figure 3). The HOMO-3 (−6.30 eV) involves the π orbital of the P−P double bond (Figure S28) and the LUMO (−1.62 eV) is mainly the π* orbital of the P−P and C−C double bonds (Figures 3, S28).
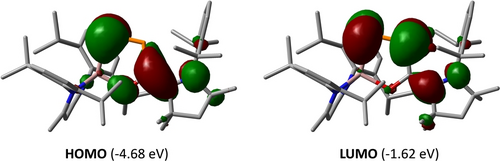
HOMO (left) and LUMO (right) of 2 calculated at the PBE0-D3BJ/def2-TZVP level of theory. The isovalue was arbitrarily chosen to be 0.03. Hydrogen atoms are omitted for clarity.
The calculated HOMO–LUMO energy gap of 2 (−3.06 eV) encouraged us to test its redox properties. The cyclic voltammogram (CV) of 2 showed a reversible reduction event (E1/2=−2.16 V, Figure S14) due to the formation of the corresponding radical anion and an irreversible event at Epa=−0.20 V (Figure S15). Indeed, [K(DB-18-c-6][LGa(P2OC)cAAC] 3[K(DB-18-c-6] containing the radical anion 3⋅− was isolated as dark violet solid from the reaction of 2 with KC8 and dibenzo-18-crown-6 (DB-18-c-6, Scheme 2). In contrast, all oxidation reactions of 2 failed and only LGa was formed.

One-electron reduction of 2 to [K(DB-18-c-6)][LGa(P2OC)cAAC] 3[K(DB-18-c-6] containing the radical anion 3⋅− and its reaction with [Fc][B(C6F5)4] to 2.
Compound 3[K(DB-18-c-6)] is stable in solution and solid state under an inert gas atmosphere but decomposes rapidly when exposed to air. The formation of the radical anion 3⋅− was confirmed by electron paramagnetic resonance (EPR) spectroscopy. The continuous wave (CW) X-band (≈9.45 GHz) EPR spectrum of 3 in fluorobenzene at 293 K shows a multiline isotropic signal centred at giso=2.0103 (Figure 4, top). The small g-shift observed from ge is similar to that of diphosphorus radicals and consistent with the unpaired electron being largely localized to the P2 unit. The multiline pattern results from the (isotropic, aiso) hyperfine interaction of the unpaired electron with the two P nuclei of the P2 core, as well as the Ga nuclei, each of which possess natural abundance NMR-active nuclear isotopes (I(31P)=1/2, 100 %; I(69Ga)=3/2, 60.11 %; I(71Ga)=3/2, 39.89 %).19 The solution CW X-band EPR spectrum is well simulated, Figure 4, with isotropic components of each tensor, giso=2.0103, and isotropic hyperfine couplings of |aiso(31P2)|=147.8 MHz, |aiso(31P1)|=51.6 MHz and |aiso(69Ga)|=38.3 MHz. We do note that the measured room temperature EPR is not completely isotropic, due to some slower motion tumbling of the molecule, however, the isotropic simulation reproduces all features and their positions well.
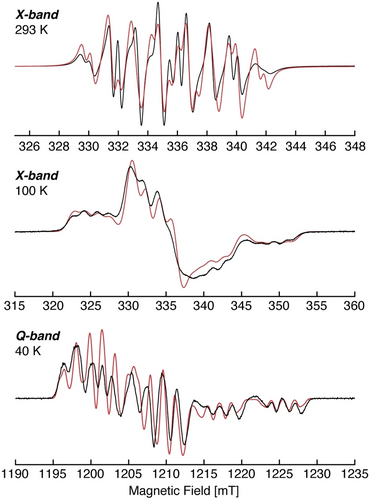
CW X-band (≈9.45 GHz) EPR spectrum of 3[K(DB-18-c-6)] in fluorobenzene at 293 K (top), frozen solution in fluorobenzene at 100 K (center), and first derivative of pulsed Q-band (≈33.97 GHz) EPR absorption spectrum of a frozen solution in THF at 40 K (bottom). Room temperature EPR simulation parameters: giso=2.0103, aiso(31P1)=51.6 MHz, aiso(31P2)=147.8 MHz and aiso(69Ga)=38.3 MHz. Frozen X- and Q-band EPR simulation parameters: g=[2.0205, 2.0080, 2.0020], A(31P1)=[−51, −53, 236] (aiso=40) MHz, A(31P2)=[22.5, −24, 441] (aiso=131.5) MHz, A(69Ga)=[−46.6, −44, −41] (aiso=−43.7) MHz. Spectrometer conditions are described in the Supporting Information.
To resolve the complete EPR spin Hamiltonian parameters, including the anisotropic character of the g-tensor and hyperfine A-tensors, multifrequency X- and Q-band EPR spectra of frozen solutions of 3⋅− were collected, Figure 4. Global fitting20 to the low temperature multifrequency EPR spectra yielded a single set of EPR parameters with complete rhombic g-tensor of g=[g1, g2, g3]=[2.0205, 2.0080, 2.0020] (giso=2.010), and hyperfine tensors determined by simulation for the two distinct phosphorus atoms of A(31P1)=[A1, A2, A3]=[−51, −53, 236] MHz (aiso=131.5 MHz) and A(31P2)=[22.5, −24, 441] MHz (aiso=40 MHz), while the hyperfine tensor for the Ga atom is A(69Ga)=[−47, −44, −41] MHz (aiso=−43.7 MHz). The absolute hyperfine values may either be inferred from the spin density distribution,11a, 21 or also aided through DFT computations (Supporting Information).22-32 The isotropic EPR g- and A-values determined from simulation of the low temperature spectra agree well with those directly simulated for the room temperature EPR spectrum. The simulated hyperfine tensors agree with the DFT calculated tensors (ADFT(31P)=[−25.4 −33.4 185] MHz and [−11.6 −26.9 408] MHz and ADFT(69Ga)=[−22.9 −27.9 −29.7] MHz), which also assigns positive aiso values for P1 and P2, and negative for the Ga atom.
The axial phosphorus tensors are consistent with the unpaired electron being localized in a π*-symmetry type orbital, although the observed maximal hyperfine values of 236 and 441 MHz are less than what has previously been reported for P2 radicals,4i, 5e-5h, 15 indicating a greater degree of delocalization of the unpaired electron onto the ligands, including the conjugated C−C double bond in 3⋅−. The π* character of the P2 radical is further revealed from the large axial anisotropic contributions, T=[−t, -t, 2 t]=A—aiso, to the hyperfine tensor: T(P2)=[−124, −170.5, +294.5] MHz, T(P1)=[−95, −97, +192] MHz. This large t component corresponds to significant but inequivalent p-orbital spin populations of ρ(p-P2) ≈0.40 and ρ(p-P1) 0.26 for the two P atoms (where the anisotropic hyperfine coupling constant is 366.8 MHz for 31P).19 This agrees with the asymmetrical structure of 3⋅− and the calculated spin density distribution, predicting that the P2 bears more spin than P1 with the unpaired electron in the P2 π* molecular orbital.
3[K(DB-18-c-6)] crystallizes in the triclinic space group P with one ion pair and solvent molecules (THF, n-hexane) in the asymmetric unit (Figure 2).17 The P1−P2 (2.1620(4) Å) bond of 3[K(DB-18-c-6)] is elongated by 10 pm compared to that of 2, in accordance with the single-electron population of the LUMO of 2 (π* orbital), but is still shorter than the sum of the calculated P−P (2.22 Å) single bond radii.18b In contrast, the C30−C31 bond (1.3805(13) Å) in the radical anion 3⋅− is slightly shorter compared to that in neutral 2 and the P2−C30 (1.8202(9) Å) slightly longer than that in 2, respectively. In addition, the C31−N3 bond (1.4259(12) Å) is slightly elongated compared to that of 2 and to the corresponding C−N bonds in the radical cation IV⋅+ (1.349(2) Å),10 while the Ga1−P1 bond (2.2418(3) Å) is slightly shorter than that of 2 (2.2844(4) Å).
with one ion pair and solvent molecules (THF, n-hexane) in the asymmetric unit (Figure 2).17 The P1−P2 (2.1620(4) Å) bond of 3[K(DB-18-c-6)] is elongated by 10 pm compared to that of 2, in accordance with the single-electron population of the LUMO of 2 (π* orbital), but is still shorter than the sum of the calculated P−P (2.22 Å) single bond radii.18b In contrast, the C30−C31 bond (1.3805(13) Å) in the radical anion 3⋅− is slightly shorter compared to that in neutral 2 and the P2−C30 (1.8202(9) Å) slightly longer than that in 2, respectively. In addition, the C31−N3 bond (1.4259(12) Å) is slightly elongated compared to that of 2 and to the corresponding C−N bonds in the radical cation IV⋅+ (1.349(2) Å),10 while the Ga1−P1 bond (2.2418(3) Å) is slightly shorter than that of 2 (2.2844(4) Å).
The PBE0-D3BJ/def2-TZVP-optimized geometry of 3⋅− agrees well with the anionic part of 3[K(DB-18-c-6)] in the solid-state structure (Figure S23).17 To gain further insight into the electronic structure of 2 and 3⋅− quantum theory of atoms in molecules (QTAIM)33 analyses were performed (Figure S27). The one-electron reduction of 2 to 3[K(DB-18-c-6)] leads to an elongation of the P1−P2 and P2−C30 bonds and a shortening of the C30−C31 bond (Figure S23), which is consistent with the decrease (P1−P2, P2−C30) and increase (C30−C31) of the calculated bond orders. These changes are most pronounced for the P1−P2 bond (Figure S26), since the π* orbital is mainly located on the electropositive P atoms. Of particular interest is the change in ellipticity at the bond critical points (Figure S27): An increase in the ellipticity and thus in the π character of the P2−C30 bond upon reduction is observed as is expected for a conjugated 1,3-butadiene system. However, the P2−C30 bond in 3⋅− is longer than that in 2, indicating that the σ bond strength decreases, most likely due to a decrease of the electrostatic interaction between the more negative P atom and the C atom. The difference of the NBO charges between P2 and C30 in 2 amounts to 0.44 e, whereas for 3⋅− a value of 0.08 e is calculated (Figure S24). In contrast, the C30−C31 bond length decreases by 2 pm and the C31−N3 bond increases by 4 pm upon reduction, which again is explained by a decrease in the electrostatic interaction between C31 and N3 by lowering the charge on C31. NBO charges show a substantial increase of the negative charge at the phosphorus atoms (Figure S24), and the NBO atomic spin-density for 3⋅− (Figures 5, S25) reveals that the unpaired electron is mainly located at the π-conjugated 1,2-diphospha-1,3-butadiene system.
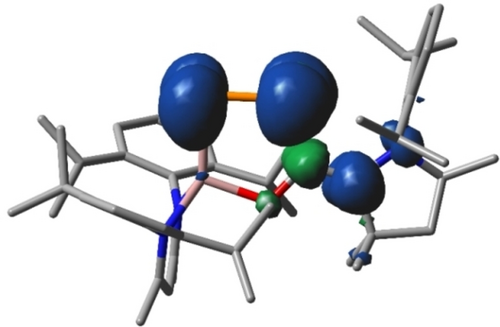
Spin density plot for 3⋅− calculated at PBE0-D3BJ/def2-TZVP level of theory (isovalue 0.002). Hydrogen atoms are omitted for clarity.
The UV/Vis spectra show two strong absorptions for 2 at 350 and 557 nm (Figure S16) and three at 425, 555, and 680 nm (Figure S18) for 3[K(DB-18-c-6)]. The spectra were simulated by TD-DFT calculations at PBE0-D3BJ(SMD,THF)/def2-TZVP level of theory (Figures S17, S19). A comparison of the calculated and measured spectra shows that only the bands at 557 nm (2) and 680 nm (3[K(DB-18-c-6)]) can be assigned to single transitions with certainty. In the case of 2, the band corresponds to the transition from HOMO to LUMO, while in the case of 3[K(DB-18-c-6)], the band at 680 nm represents the transition of the β-electron from HOMO-1 to SOMO. In terms of the orbital model, both are transitions from π2 to π3 of a butadiene system, differing only in energy. Due to the reduction, the HOMO-1 of 3[K(DB-18-c-6)] (π2) is energetically higher than the HOMO of 2 (π2), which leads to the red shift of the transition.
The chemical reactivity of 3[K(DB-18-c-6)] was investigated in its reaction with [Fc][B(C6F5)4]. 3[K(DB-18-c-6)] was quantitatively reoxidized to 2 (Scheme 2) according to 1H (Figure S6) and 31P NMR (Figure S7) spectroscopy. In addition, reaction of 3[K(DB-18-c-6)] with TEMPO at −30 °C occurred with H abstraction of a β-diketiminate Me group and internal electron transfer from the 1,2-diphospha-1,3-butadiene π-system to the conjugated diketiminate ligand system to give [K(DB-18-c-6][L−HGa(P2OC)cAAC] 4[K(DB-18-c-6 containing the 1,2-diphospha-1,3-butadiene anion 4− (Scheme 3). Heating this reaction solution to 110 °C resulted in quantitative oxidation of 4[K(DB-18-c-6)] to 2 as demonstrated by variable temperature (VT) 1H and 31P NMR spectroscopy (Figure S12–S13). The 31P{1H} NMR spectrum of 4[K(DB-18-c-6)] shows two doublets at 486.9 ppm (d, 1JPP=595 Hz, Ga−P=P), 243.3 ppm (d, 1JPP=595 Hz, Ga−P=P) for the magnetically inequivalent P atoms.
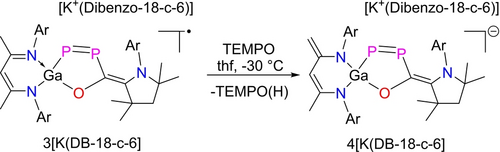
Reaction of 3[K(DB-18-c-6)] with TEMPO yielding [K(DB-18-c-6)][L−HGa(P2OC)cAAC] 4[K(DB-18-c-6.
4[K(DB-18-c-6)] crystallizes in the monoclinic space group C2/c with one ion pair plus solvent molecules (toluene) in the asymmetric unit (Figures 2 and S22).17 The P1−P2 (2.0653(7) Å), P2−C30 (1.787(2) Å) and C31−N3 (1.373(3) Å) bonds of 4[K(DB-18-c-6)] are shortened and the C30−C31 (1.394(3) Å) and Ga1−P1 (2.3068(6) Å) bonds are elongated compared to those of 3[K(DB-18-c-6)], but comparable to those of 2, suggesting similar electronic structures of the P2C2N units in the neutral molecule 2 and the anion 4− (Figures S23–27), as was confirmed by quantum chemical calculations. However, in contrast to compound 2, the HOMO of 4− is localized at the β-diketiminate ligand (Figure S28).
Conclusion
Compound 2 containing the 1,2-diphospha-1,3-butadiene moiety and its radical anion 3⋅− were structurally characterized. The unpaired electron in 3⋅− is asymmetrically delocalized over the π-conjugated P2C2 unit according to DFT and EPR spectroscopic studies. 3[K(DB-18-c-6)] reacts with [Fc][B(C6F5)4] with regeneration of 2, while its reaction with TEMPO gave the 1,2-diphospha-1,3-butadiene anion 4−. Our results show that LM-substituted bis-phosphaketenes13b are promising starting reagents for the isolation of P-containing π-conjugated open-shell molecules. Since many singlet carbenes with tunable donor-acceptor properties are known, alternative 1,2-diphospha-1,3-butadiene units with different electronic properties and their corresponding radical ion salts may be isolated in the future. The five-membered GaP2OC heterocyclic ring may also be opened to give novel 1,2-diphospha-1,3-butadiene derivatives with different electronic situation and reactivity.
Acknowledgments
Financial support from the Deutsche Forschungsgemeinschaft (SCHU 1069/27-1, INST 20876/282-1 FUGG), the Max-Plank Society (G.E.C.) and the University of Duisburg-Essen (S.S., G.H.) is acknowledged. We also acknowledge support by the Open Access Publication Fund of the University of Duisburg-Essen. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




