Harnessing Aromatic-Histidine Interactions through Synergistic Backbone Extension and Side Chain Modification
Abstract
Peptide engineering efforts have delivered drugs for diverse human diseases. Side chain alteration is among the most common approaches to designing new peptides for specific applications. The peptide backbone can be modified as well, but this strategy has received relatively little attention. Here we show that new and favorable contacts between a His side chain on a target protein and an aromatic side chain on a synthetic peptide ligand can be engineered by rational and coordinated side chain modification and backbone extension. Side chain modification alone was unsuccessful. Binding measurements, high-resolution structural studies and pharmacological outcomes all support the synergy between backbone and side chain modification in engineered ligands of the parathyroid hormone receptor-1, which is targeted by osteoporosis drugs. These results should motivate other structure-based designs featuring coordinated side chain modification and backbone extension to enhance the engagement of peptide ligands with target proteins.
Introduction
Biology relies on information encoded in polypeptide sequences for the transmission of messages within and between cells. Efforts to modify these messages have traditionally explored changes in one side chain or more within the original information-bearing peptide.1 This approach is inspired by the central biological mechanism for exploring variations in information content (i.e., mutation). However, chemical synthesis and, more recently, rational engineering of biosynthetic machinery have allowed the exploration of a much larger side chain range than is conventionally accessible in ribosomally generated polypeptides.2 In addition, D-α-amino acid residues can be incorporated via chemical synthesis, with favorable outcomes in some cases.3
Non-natural synthetic strategies enable modification of the peptide backbone, e.g., introduction of additional atoms between carbonyl and nitrogen of an amino acid residue, an approach that is distinct from altering the side chain or configuration of an α-amino acid residue.4 The impact of backbone extension on biological recognition has received less attention relative to side chain or configuration alterations.5 Backbone extension can be achieved, for example, by replacing an α-amino acid residue with a β-amino acid residue, which adds one carbon atom to the backbone.6 Other approaches feature addition of two or three backbone atoms to a single subunit, relative to an α-amino acid residue.4c, 5d-5f, 5h, 5j Pursuit of backbone extension strategies has often been motivated by the goal of suppressing proteolysis near the modification site.7 Results from diverse systems indicate that the recognition characteristics of a natural all-α sequence can be retained after backbone extension, but affinity for the partner protein is often diminished relative to the natural prototype.8
The work described here was motivated by the hypothesis that the stability of a ligand-receptor complex could be enhanced by introducing a stabilizing intermolecular side chain-side chain interaction that does not occur in the natural complex. Specifically, we sought to modify the parathyroid hormone-related polypeptide (PTHrP) to enhance binding to the parathyroid hormone receptor 1 (PTHR1) by introducing an aromatic side chain that could interact favorably with a native His side chain in the receptor.9 The PTHR1 is a class B G protein-coupled receptor (GPCR) that is targeted by the peptide drugs teriparatide and abaloparatide for treatment of osteoporosis; abaloparatide is a synthetic analogue of PTHrP(1–34).10 This receptor represents an excellent system for the fundamental studies described here because assays for measuring peptide binding and functional outcomes are readily available.
Our findings revealed that coordinated side chain modification and backbone extension was required to enable stabilizing interactions between an aromatic side chain on the peptide and a native His residue on the PTHR1 extracellular domain (ECD). To our knowledge, these findings represent the first demonstration that side chain modification and backbone extension can function synergistically to achieve a design goal that is not accessible via side chain alteration alone. Our ability to engage two distinct His residues on the receptor surface, and the frequent occurrence of His residues on protein surfaces, suggest that the design strategy introduced here may be broadly applicable for enhancing peptide-protein affinity.
Results and Discussion
The crystal structure of PTHrP(15–36) bound to the PTHR1 ECD (PDB 3H3G) reveals that the side chain of peptide Ile31 packs against the ECD surface in the vicinity of receptor His114; however, there is no direct contact between these side chains (Figure 1a).10a We hypothesized that affinity for the PTHR1 could be enhanced by introduction into the PTHrP sequence of an aromatic side chain positioned to contact receptor His114.
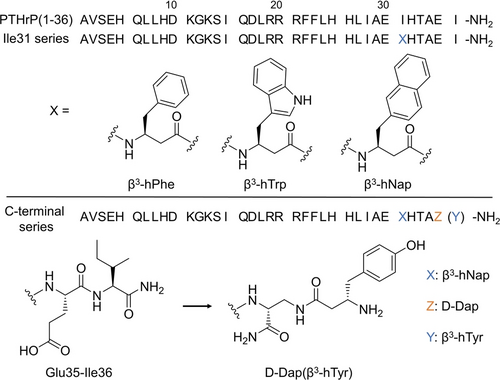
Sequences of native PTHrP and modified PTHrP peptides. Upper panel: the chemical structures of the β3 aromatic amino acids for Ile31 series; Lower panel: chemical structures of the C-terminus of the selected peptide to illustrates the replacement of the native Glu35-Ile36 segment with D-Dap(β3-hTyr) for C-terminal series.
For the initial test of our hypothesis, we employed a fluorescence polarization (FP) assay to detect the binding of fluorescein-labeled derivatives of PTHrP(15–36) to the isolated PTHR1 ECD (D29-L187). The fluorophore was linked to the N-terminus via a β-homoglycine (β-hGly) spacer. The reported co-crystal structure containing PTHrP(15–36) shows that Ile15 does not contact the ECD; therefore, we predicted that this fluorophore placement would have little effect on binding to the isolated ECD. Since all of the PTHrP(15–36) derivatives we examined had the same fluorescein placement, any influence on binding should be comparable across the series.
The FP assay indicated KD=1.8 μM for binding of the native PTHrP(15–36) peptide to the ECD (Table 1 and Figure S1). This measurement aligns well with the reported affinity of this peptide for a fusion protein containing the PTHR1 ECD linked to maltose-binding protein.10a We pursued side chain modification to try to induce a favorable His-aromatic interaction at the peptide-protein interface. However, replacing Ile31 with either Trp or 2-naphthylalanine (Nap) led to a decrease in affinity for the ECD (≈5-fold increase in KD relative to the native sequence). We therefore examined coordinated side chain modification and backbone extension at residue 31. Replacement of Ile31 with β3-hPhe had no effect on affinity for the ECD. Larger aromatic side chains, however, provided favorable results, with ≈3-fold improvement for β3-hTrp and ≈4-fold improvement for β3-hNap in affinity for the ECD, relative to PTHrP(15–36).
Residue 31 |
WT KD(μM) |
H114A KD(μM) |
|---|---|---|
I (native) |
1.8±0.2 |
1.9±0.3 |
W |
10±2.4 |
21±2 |
Nap |
11±0.6 |
19±1 |
β3-hF |
2.0±0.1 |
4.5±0.3 |
β3-hW |
0.67±0.06 |
4.8±0.2 |
β3-hNap |
0.44±0.03 |
4.7±0.3 |
- [a] Dissociation constants for FITC-labeled PTHrP(15–36) variants with indicated substitution at position 31 binding to the native PTHR1-ECD or the H114A variant, measured by fluorescence polarization in 10 mM tris-HCl (pH 7.4) containing 150 mM NaCl and 0.1 % Pluronic F-68.
To test the hypothesis that these binding improvements result from the intended His-aromatic interaction, we conducted a second set of FP assays with the His114-to-Ala variant of the PTHR1 ECD (Table 1 and Figure S1). H114A modification had no effect on the binding of the native fragment, PTHrP(15–36), which is consistent with lack of contact between the side chains of peptide Ile31 and receptor His114 in the crystal structure of the PTHrP(15–36)+ECD complex.10a Our peptides containing β3-hTrp or β3-hNap at position 31 bound ≈7-fold or ≈10-fold less strongly to the ECD H114A variant, respectively, relative to the wildtype ECD. We interpret these declines in affinity for the ECD H114A variant to reflect the loss of favorable His-aromatic interactions. Collectively, these data for binding to the native and H114A forms of the ECD suggest that replacement of Ile31 in the PTHrP sequence with a β3 residue bearing a large aromatic side chain introduces a favorable His-aromatic interaction at the peptide-protein interface.
We asked whether the beneficial effects of replacing Ile31 with a β3 residue bearing a large aromatic side chain are evident in the binding of an agonist derived from PTHrP(1–36) to the full-length human PTHR1. A recently developed assay in which binding is detected by bioluminescence resonance energy transfer (BRET) was employed.11 The native Glu35 of each peptide was replaced with tetramethyrhodamine-Lys (KTMR), and binding was evaluated with a version of the PTHR1 bearing an N-terminal nanoluciferase (nLuc) fusion. Peptide binding brings the nLuc and TMR units into proximity, enabling BRET. Rate constants for agonist association (kon) and agonist dissociation (koff) can be measured, which allows the determination of KD.
Binding of PTHrP(1–36) to the full-length receptor was much stronger than binding of PTHrP(15–36) to the ECD (KD ≈26-fold smaller in the former case; Table 2), but the trends from the two binding assays were very similar. Thus, both PTHrP(1–36) analogs containing only side chain modification at position 31 (Ile→Trp or Nap) displayed a significant decline in affinity relative to the native sequence. However, when these side chain modifications were made in concert with backbone extension (Ile31→β3-hTrp or β3-hNap), binding to the receptor improved by 3- to 4-fold relative to binding of PTHrP(1–36) itself. The rate constant data indicated that the benefit of coordinated side chain modification and backbone extension is manifested primarily in a smaller koff (Table 2 and Figure S2).
Residue 31 |
kon (x 105 s−1 M−1) |
koff (s−1) |
KD (nM) |
|---|---|---|---|
I (native) |
32±4 |
0.022±0.002 |
68±9 |
W |
23±2 |
0.063±0.014 |
273±61 |
Nap |
22±4 |
0.057±0.011 |
259±50 |
β3-hF |
46±10 |
0.023±0.002 |
50±11 |
β3-hW |
41±7 |
0.0086±0.003 |
21±4 |
β3-hNap |
28±3 |
0.0039±0.0004 |
14±2 |
- [a] Rate constants for association and dissociation and dissociation constants of TMR-labeled PTHrP(1–36) variants with indicated substitution at position 31 to the nLuc-PTHR1 on the surface of HEK293 cells measured with a BRET assay.
We asked whether the intended side chain interactions occur between the most promising PTHrP(1–36) variants and the receptor by determining co-crystal structures of the two corresponding PTHrP(15–36) derivatives, Ile31→β3-hTrp and Ile31→β3-hNap, bound to the ECD (Figure 2). A truncated variant of the ECD (D29-L187, ΔS61-R102) that lacks an internal disordered segment was used to promote co-crystallization with the modified peptides. These new structures allowed direct comparison with the previously reported structure of the PTHrP(15–36)+ECD complex.10a Both modified peptides bind to the ECD in a manner that is similar to the binding of the native fragment (Figure S3). The new structures revealed the predicted histidine-aromatic interactions between the side chain of β3-hTrp or β3-hNap of the modified peptides and side chain of receptor His114 (Figure 2). The plane of the imidazole ring displays a similar tilt relative to the plane of the indole or naphthalene unit (Figure 2b); such tilting is commonly observed between proximal aromatic side chains in proteins.12

Structural insights on interactions between side chains at position 31 of PTHrP derivatives and His114 of the PTHR1 ECD. a) Comparison of the native ligand (Ile31; PDB 3H3G; left) with the Ile31–>β3-hTrp variant (PDB: 8D51; middle) and the Ile31–>β3-hNap variant (PDB: 8D52; right). b) Angles between the plane of the His114 imidazole ring and the plane of the indole unit of β3-hTrp or the naphthyl unit of β3-hNap. c) Distances between the His114 imidazole ring center and the ring centers of the indole unit of β3-hTrp or the naphthyl unit of β3-hNap. d) Alternative perspective for the His114-β3-hTrp31 and His114-β3-hNap interactions. Although a specific orientation of the His114 imidazole unit is shown in each image, the electron density does not allow us to determine which orientation is present (i.e., which ring atoms are carbon and which are nitrogen); it is possible that both orientations are populated.
To determine whether the increased affinity achieved with coordinated side chain and backbone modification influences signaling mediated by the PTHR1, receptor activation was assessed with HEK293 cells that overexpress the PTHR1 and the GloSensor,13 which allows detection of intracellular production of the second messenger cAMP via luminescence. Agonist engagement of the PTHR1 stimulates cAMP production via the heterotrimeric GS protein. The variants of PTHrP(1–36) containing β3-hPhe, β3-hTrp or β3-hNap in place of the native Ile31 were indistinguishable from PTHrP(1–36) itself in terms of EC50 (0.3–0.4 nM) and maximum cAMP production (Figure S4). However, significant differences were observed in terms of duration of cAMP response. This parameter was assessed in “washout” experiments: cells were treated with 1 nM PTHrP(1–36) or 1 nM of an Ile31→β3 variant for 10 min, and then the medium was removed, the cells were rinsed, and cAMP production was monitored for 3 hr (Figure 3). The agonists were compared in terms of area under the curve after washout. Signaling by PTHrP(1–36) was quite transient, as has been previously reported.14 Signaling by the Ile31→β3-hPhe analogue was transient, too. The Ile31→β3-hTrp and Ile31→β3-hNap analogues, however, showed significant increases in duration of the cAMP response relative to PTHrP(1–36). We attribute these longer signaling durations to the smaller koff for these two variants relative to the native agonist. The timing of PTHR1 activation induced by externally administered agonists influences the physiological effects mediated by this receptor,15 and the ability to modulate agonist duration of action could prove useful in future efforts to improve therapeutic agents for osteoporosis.
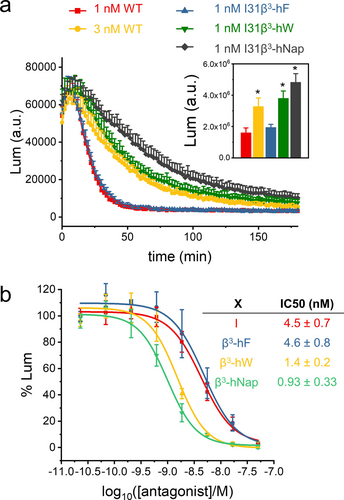
Agonism and antagonism of PTHrP-derived peptides containing β-amino acid residue replacements for Ile31. a) Results of washout experiments (described in the text) with HEK293 cells expressing the PTHR1 and GloSensor. Luminescence reflects cAMP production as a function of time after agonist washout. Inset: average and standard deviation for area under curve from three independent experiments (* relative to 1 nM WT data, p<0.05, analyzed by T-test) b) Inhibition of cAMP formation in SaOS-2 cells expressing PTHR1 and GloSensor stimulated by 1 nM PTHrP(1–36) as a function of antagonist concentration for antagonist peptides with sequences FMHNLwKHLSDLRRRFFLHHLIAEXHTAEI, where X is Ile (corresponding to Ile31 in PTHrP) or a β-amino acid residue, and w is D-Trp.
Excessive activation of the PTHR1 can cause adverse outcomes, including hypercalcemia and bone degradation.16 This problem can arise from overproduction of PTH or PTHrP, and the development of antagonists of PTHR1 signaling has therefore been of biomedical interest.17 One of the most potent known antagonists is derived from residues 7–34 of bovine PTH, with the native Gly12 replaced by D-Trp.18 We designed an all-α antagonist peptide containing the first 10 residues of bPTH(7–34)-D-Trp12, followed by residues corresponding to PTHrP(17–36) (sequence in the caption of Figure 3a) in order to test our hypothesis that α→β3 replacements at a site corresponding to Ile31 of PTHrP would enhance antagonist potency.
Antagonism assays were conducted with SaOS-2 cells expressing the GloSensor; in contrast to the HEK293 cell system, SaOS-2 cells express the PTHR1 at native levels. When 1 nM PTHrP(1–36) was used to activate the receptor, and varying amounts of the antagonist containing Ile31 were included, inhibition of cAMP production was observed with IC50=4.5 nM (Figure 3b) The Ile31→β3-hPhe analogue displayed a similar IC50, but the Ile31→β3-hTrp and Ile31→β3-hNap analogues were more potent as antagonists, by ≈3-fold and ≈4-fold, respectively. These improved inhibitor potencies align with the enhanced affinities displayed by the corresponding analogues of PTHrP(15–36) for the ECD and the corresponding analogues of PTHrP(1–36) for the full-length PTHR1. Thus, the antagonism studies provide further support for the conclusion that coordinated side chain and backbone extension at position 31 of PTHrP enables a new attractive interaction at the peptide-receptor interface between the His114 side chain and the aromatic side chain of β3-hTrp or β3-hNap.
The PTHR1 ECD contains a second surface His residue, at position 160, which is near but not in contact with PTHrP(15–36) in the co-crystal structure. We hypothesized that modifying the last two residues of the peptide (Glu35-Ile36) would enable a favorable His-aromatic contact. However, replacing either Glu35 or Ile36 with a β3 residue bearing an aromatic side chain did not result in enhanced affinity for the ECD (data not shown). We therefore explored a different backbone-modification strategy in which Glu35 was replaced with an L- or D-2,3-diaminopropionic acid (Dap) residue, and the β amino group of Dap was acylated with an α- or β-amino acid bearing an aromatic side chain. An initial FP-based screen suggested that a D-Dap+L-β3 combination was most favorable (Figure S5). These peptides were prepared by attaching Fmoc-Dap(Alloc)-OH to Rink amide resin, using standard Fmoc-based methods to add the next 34 residues, removing the Alloc protecting group, adding the Fmoc-protected β3-amino acid, removing the Fmoc protecting group, and cleaving the peptide from the resin.
A series of PTHrP(15–36) derivatives containing the Ile31→β3-hNap and Glu35→D-Dap modifications and various C-terminal residues was evaluated via FP assays for affinity for the native PTHR1 ECD and the His160-to-Ala variant (Table 3). β3-hPhe at the terminal position led to a small increase in affinity for the native ECD relative to the native Glu35-Ile36 segment, but this modification led to decreased affinity relative to Glu35-Ile36 for the H160A ECD variant. Replacing β3-hPhe with either Phe or β3-hAla caused weaker affinity for the native ECD, suggesting a synergy between the extended backbone and aromatic side chain in the terminal residue. β3-hTyr was the most favorable residue at the C-terminal position, providing ≈3-fold higher affinity relative to the native Glu35-Ile36 segment. This benefit was lost with the H160A variant of the ECD. Enlarged the aromatic side chains, from β3-hTrp or β3-hNap, decreased affinity for the ECD.
Residue 31 |
C-terminus |
WT KD(μM) |
H160A KD(μM) |
|---|---|---|---|
β3-hNap |
Glu-Ile |
0.44±0.06 |
0.48±0.08 |
β3-hNap |
D-Dap(β3-hPhe) |
0.26±0.03 |
1.1±0.2 |
β3-hNap |
D-Dap(Phe) |
1.9±0.2 |
1.7±0.3 |
β3-hNap |
D-Dap(β3-hAla) |
1.0±0.2 |
1.2±0.1 |
β3-hNap |
D-Dap(β3-hTyr) |
0.15±0.2 |
1.3±0.2 |
β3-hNap |
D-Dap(β3-hTrp) |
0.32±0.04 |
0.81±0.12 |
β3-hNap |
D-Dap(β3-hNap) |
0.35±0.05 |
1.0±0.1 |
- [a] Dissociation constants for FITC-labeled PTHrP(15–36) variants with indicated substitutions at position 31 and C-terminus binding to the native PTHR1-ECD or the H160A variant, measured by fluorescence polarization in 10 mM tris-HCl (pH 7.4) containing 150 mM NaCl and 0.1 % Pluronic F-68.
The C-terminal modifications were further evaluated in the context of PTHrP(1–36) derivatives binding to the nLuc-PTHR1. Direct BRET measurements were not possible because the C-terminal modifications prevented placement of the necessary KTMR residue. We therefore used a competition BRET assay involving displacement of the tracer PTHrP(1–36, E35KTMR). The trends in this series mirrored the trends observed for binding to the native ECD (Table 4). The peptide containing Ile31→β3-hNap along with the best C-terminal modification, D-Dap-β3-hTyr, had ≈8-fold lower KD relative to PTHrP(1–36). This outcome suggests that two different surface His residues on a target protein can be productively engaged with a single modified sequence.
Residue 31 |
C-terminus |
IC50(nM) |
|---|---|---|
Ile |
Glu-Ile |
80±2 |
β3-hNap |
Glu-Ile |
20±3 |
β3-hNap |
D-Dap(β3-hPhe) |
15±2 |
β3-hNap |
D-Dap(Phe) |
59±7 |
β3-hNap |
D-Dap(β3-hAla) |
43±4 |
β3-hNap |
D-Dap(β3-hTyr) |
9.7±1.1 |
- [a] A BRET competition assay was performed to measure the inhibition of wt PTHrP(1–36) binding to nLuc-PTHR1 by modified PTHrP variants. A solution containing 70 nM wt PTHrP(1–36, E35KTMR) and various concentrations of unlabeled PTHrP (1–36) variants with indicated substitutions were added to cells expressing nLuc-PTHR1, followed by measurement of BRET. The substitutions at residue 31 and/or C-terminus of peptide variants were highlighted in the table. All values shown were the average from triplicates and STDs were calculated.
The variant of PTHrP(1–36) containing Ile31→β3-hNap and D-Dap-β3-hTyr at the C-terminus was a potent agonist of the PTHR1, as indicated by HEK293 cell assays for cAMP production; EC50 was similar to that of PTHrP(1–36) or the Ile31→β3-hNap variant (Figure S8). However, the duration of the cAMP response stimulated by the fully modified peptide was significantly enhanced relative to the durations for PTHrP(1–36) or the Ile31→β3-hNap variant (Figure 4). Thus, the enhanced affinity achieved by targeting two surface His residues simultaneously appears to prolong the duration of signaling.
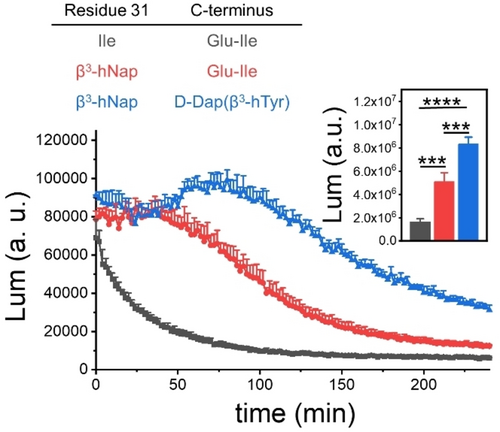
Washout experiments with HEK293 cells expressing the PTHR1 and GloSensor. 1 nM native PTHrP or PTHrP-derived peptides containing β-amino acid residue replacements for Ile31 and/or C-terminus as agonist was used to stimulate activation of receptors. Luminescence reflects cAMP production as a function of time after agonist washout. Inset: average and standard deviations for area under curve from n≥3 experiments (***p<0.001, ****p<0.0001, analyzed by T-test).
Analysis via circular dichroism (CD) has shown that PTHrP(1–34)-NH2 is largely unfolded in aqueous solution in samples that are sufficiently dilute to avoid self-association (10 μM), but addition of 2,2,2-trifluoroethanol (TFE) induces partial α-helicity.19 We used the previously identified conditions to examine PTHrP(1–36) via CD, and the results paralled those reported for PTHrP(1–34) (Figure S9). CD studies of the PTHrP(1–36) variant containing the Ile31→β3-hNap modification, and the variant containing the Ile31→β3-hNap and C-terminal D-Dap(β3-hTyr) modifications led to similar conclusions (Figure S9). Thus, all three peptides are largely unfolded in aqueous buffer. Since PTHrP is fully helical when engaged by the PTHR1,20 we conclude that binding to the receptor induces helix formation for all of the peptides discussed here.
Conclusion
The findings described here support the hypothesis that new aromatic-histidine interactions between a peptide ligand and a protein partner can be achieved via coordinated implementation of backbone extension and side chain modification and result in enhanced peptide-protein affinity. Favorable outcomes were observed in efforts directed toward two His residues on the surface of the PTHR1. Since histidine is common on protein surfaces, engineered His-aromatic interactions may prove to be a general strategy for strengthening peptide-protein binding via rational design.
Backbone modifications in the new peptides developed for this study are localized within the C-terminal portion and limited in extent; therefore, it is very unlikely that the new analogues would display meaningful resistance to proteolysis. Teriparatide (PTH(1–34)) is rapidly degraded in tissue homogenates, with a major cleavage site toward the center of the sequence;21 protection from this cleavage is not anticipated for the peptides we describe. However, future studies could combine the His-targeting approach introduced here with design strategies aimed at maximizing the extent of backbone modification in order to achieve meaningful protection from proteolysis.5d-5f, 22
Acknowledgments
This work was funded in part by the NIH grant R01GM056414. We are grateful to the Vilas Fund for partial support. We thank Weiting Lyu for help with preliminary studies. Crystal handling was performed in the Collaborative Crystallography Core at UW-Madison. Advanced Photon Source supported by the U.S. Department of Energy, Basic Energy Sciences, Office of Science, under contract No. DE-AC02-06CH11357. LS-CAT Sector 21 was supported by the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor (Grant 085P1000817). Use of LS-CAT was supported by the UW-Madison Office of VCRGE. The Center for BioMolecular Structure (CBMS) is primarily supported by the National Institutes of Health, National Institute of General Medical Sciences (NIGMS) through a Center Core P30 Grant (P30GM133893), and by the DOE Office of Biological and Environmental Research (KP1605010). This research used resources 17-ID-1 or 17-ID-2 of the National Synchrotron Light Source II, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704.
Conflict of interest
S.H.G. is a co-founder of Longevity Biotech, Inc., which is pursuing biomedical applications of α/β-peptides.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




