Electrode Materials for Desalination of Water via Capacitive Deionization
Abstract
Recent years have seen the emergence of capacitive deionization (CDI) as a promising desalination technique for converting sea and wastewater into potable water, due to its energy efficiency and eco-friendly nature. However, its low salt removal capacity and parasitic reactions have limited its effectiveness. As a result, the development of porous carbon nanomaterials as electrode materials have been explored, while taking into account of material characteristics such as morphology, wettability, high conductivity, chemical robustness, cyclic stability, specific surface area, and ease of production. To tackle the parasitic reaction issue, membrane capacitive deionization (mCDI) was proposed which utilizes ion-exchange membranes coupled to the electrode. Fabrication techniques along with the experimental parameters used to evaluate the desalination performance of different materials are discussed in this review to provide an overview of improvements made for CDI and mCDI desalination purposes
1 Introduction
Over the years, we have seen unprecedented water contamination from human activities; a decrease in groundwater levels, and overconsumption of fresh water because of the growing population. The culmination of these developments led to an alarming situation of fresh water scarcity around the globe.1 To address the water scarcity issue, there is a great interest in the development of water desalination technologies to convert sea and waste-water into fresh water for human consumption, agriculture, and industries. Desalination provides a low-cost method of producing drinking water from saline and brackish natural waters.2 Seawater desalination is the most widely used approach in mitigating water shortage, particularly for potable water needs. However, inland desalination faces challenges in disposing of desalination byproducts (brine), which leads to significant costs for water extraction.2 Nonetheless, rising desalinated water recovery rates from novel techniques like membrane distillation (MD)3 should result in lower disposal costs. Because of the varying salt concentrations in both sea and brackish water sources, a wide array of desalination technologies exist and some of them are in advanced stages of development.4-8
While selecting the best-suited technique to treat a specific type of water, factors such as water quality, salinity, fouling, and scaling have to be considered. In general, reverse osmosis (RO),4 nanofiltration (NF),5 ultrafiltration (UF),6 and microfiltration (MF)7 are examples of pressure-driven procedures where RO and NF demonstrate impressive desalination proficiency.8 Contrarily, high-efficiency salt removal can be achieved using techniques such as electrodialysis (ED) and electrodialysis reversal (EDR),9 however, their utility has been restricted thus far due to high cost, time consumption, difficult processability, and substantial energy consumption. Therefore, at present, there is an urgent need to undertake research measures to help these technologies to be made more economically viable and cost-effective.10-13
Notably, capacitive deionization (CDI) has been identified as an economic, cost-effective, and robust alternative technology for the desalination of sea and wastewater with minimal salt contamination (below 10 g L−1).14, 15 CDI cell system consists of current collectors, and porous electrodes (static or flow electrode) as sorption media separated by either an open channel or a separator made up of a dielectric material.16 On application of a voltage across the two electrodes, due to potential difference, an electric field is generated between the electrodes. As a result, the ions get temporarily immobilized between the counter electrodes and consequently get stored electrostatically in the electrical double layers (EDLs) formed at the electrode/water interface to generate a fresh water stream.16 The EDLs consist of the stern layer and the diffuse layer: the former is firmly bound to the electrode surface whereas the latter is loosely bound to the first layer (Figure 1). In the stern layer, ions are strongly attracted to the surface of the electrode due to electrostatic forces. The diffuse layer is formed by the ions that are not strongly bound to the surface, but are still in the vicinity of the electrode due to Coulombic interactions with the stern layer. The thickness of the EDL depends on various factors such as the electrode material, pore size, and electrolyte concentration. Absorbed ions are flushed out via either polarity reversal or short circuit to regenerate the electrode for a fresh cycle of water desalination. During this process, the adsorbed ions are released back into the bulk solution generating a stream of brine solution concomitantly.
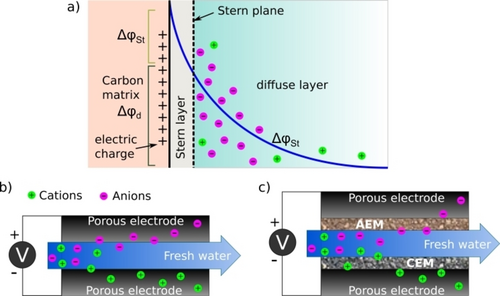
Schematic representation of a) EDL, b) CDI, and c) mCDI system.
Generally, CDI is preferred over other technologies because of low energy consumption to remove salt ions while distillation technologies demand large input of energy to desalinate water.10 Furthermore, the electrodes used in CDI technologies are recyclable: the absorbed ions during the desalination of water can be desorbed from the electrodes and the electrodes can be reused. In addition, a possible recovery of valuable ion contaminants desorbed during electrode regeneration suggests the value of CDI technology to fit into the circular water economy. Overall, the CDI meets the eligibility criteria of cost-effective and environment-friendly technology for desalination.
Although CDI is a promising technology for water desalination and there have been increasing attention in recent years, detailed information to aid in the understanding of electrode materials used in CDI technologies is still lacking in the literature. In this review, we summarize the fundamentals and different cell architectures designed for CDI/mCDI desalination process. Furthermore, we cover several electrode materials and categorized them into a) organic, b) inorganic, c) polymer, and d) bio-based materials. Several parameters often utilized to characterize the mCDI water desalination performance of the electrode materials are also discussed. In a separate section, we described the role of carbon-derived materials fabricated as ion-exchange membranes in mCDI applications. Furthermore, we have dedicated a section for future prospects, where we have discussed various strategies that may be utilized to improve the water desalination performance of the materials used in CDI/mCDI.
2 CDI cell architectures
In early 1960, Blair and Murphy introduced the concept of electrochemical demineralization.17 Later in 1996, for the first time, Farmer et al. demonstrated the electrosorption removal of NaCl and NaNO3 from water solution by utilizing carbon aerogel electrodes.18 Since then, a colossal amount of research has been performed to further develop novel CDI cell architectures including electrode materials with improved CDI performance (Figure 2).12
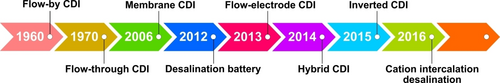
Chronological progress of various CDI cell architectures designed for desalination of water in last several decades.12
The CDI system is designed to allow the charged ionic species present in aqueous solutions to be easily removed through electrostatic or electrochemical interactions. A conventional CDI cell system is comprised of two carbon electrodes separated by open channel and feed water stream flow parallel in direction to the assembled electrodes alignment (Figure 3a).16 First in the chronological development of CDI cell architecture is the flow-by (FB)-CDI setup consisting two graphite current collectors, porous carbon-based electrodes for absorbing ions, and open space channel to feed water transportation. Due to the flow of feed water between the electrodes, the FB-CDI architecture is also known as flow-between CDI,11, 17 which has been widely explored to investigate the water softening, salt ion removal, and electrosorption performance of novel materials developed as electrode material in the water desalination application. Soon after, the flow-through (FT)-CDI architecture was introduced that consists of two electrodes separated by a porous separator, which acts as a barrier to prevent the mixing of ion-enriched and ion-depleted water streams, ensuring that the purified water is collected separately from the waste stream. The separator also allows efficient mass transport of ions to and from the electrodes, thereby increasing the efficiency of the deionization process. The porous separator also traps small particles and organic substances that might be present in the wastewater to prevent clogging of the electrodes (Figure 3b).19 In FT-CDI, the feed water stream flows in a direction perpendicular to the assembled electrodes. Since a separator is not a major feed water pathway in FT-CDI, a decrease in the thickness of the separator offers lower cell ionic resistance which results in a high desalination rate and high salinity feeds per charge, compared to FB-CDI.20
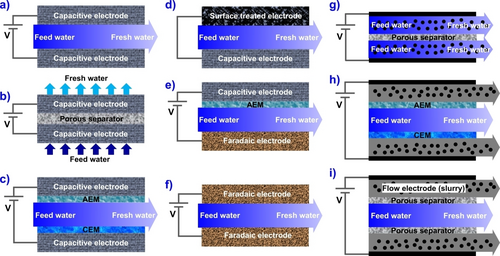
Types of CDI cell architectures: a) flow-between electrodes, b) flow-through electrode, c) membrane CDI, d) inverted CDI, e) desalination battery, f) hybrid CDI, g) feed-in electrodes, h) feed-between electrodes, and i) membrane flow electrode.12
The desalination process in both FB-CDI and FT-CDI systems is diffusion controlled. However, due to large feed pressures, the FT-CDI often exhibits longer desalination time compare to the FB-CDI system.21 Employing electrode materials possessing large macropores with diameter >1 μm and micropores of nanometer size are beneficial for flow-through operation.22, 23 Surprisingly, it has been observed that the oxidation of the anode is faster in the FT-CDI cell system compared to FB-CDI. To eliminate this issue, nitrogen purging in the feed to remove dissolved oxygen and chemical treatments of the electrode surface has been shown to increase the lifetime and desalination performance of the FT-CDI cell system.22, 24
In general, a “co-ion expulsion” effect was found responsible to hamper the salt ion removal capacity performance of the CDI architecture. This phenomenon involves degradation of the electrodes due to direct contact with feed water stream.25 Generally, in deionization process, both adsorption and desorption of ions occur simultaneously and therefore, the charge efficiency (defined as the ratio of salt absorbed over the charge) of the CDI system decreases and in turn increases the energy consumption.26 To alleviate such issue, Lee et al. demonstrated a strategy based on placing ion exchange membranes (IEMs) in front of both anode and cathode (Figure 3c).27 Such architecture is known as membrane capacitive deionization (mCDI) cell system. Notably, by placing IEMs in the mCDI cell system, the co-ions are expelled from micropores but housed by the macropores while maintaining electrical neutrality. These macropores provide kind of extra space for co-ion storage that strictly restricts the co-ions from carrying the parasitic current and ultimately, improves the salt ion removal performance of the IEMs in the water desalination, which is one of the major advantages associated with mCDI over conventional CDI cell system. Typically, the mCDI system consists of free-standing cation-exchange membrane (CEM) placed at cathode and free-standing anion exchange membrane (AEM) placed at the anode.27 However, instead of utilizing free-standing membranes, the membrane of varying thickness can be coated directly onto the porous electrodes.
Due to direct contact of electrode with water, the formation of oxides such as carboxylic groups at the surface of the electrode via oxidation is a common phenomenon that causes performance deterioration during CDI desalination process.28 To achieve sustainable desalination performance, an inverted-CDI (i-CDI) (modified version of the FB-CDI) was introduced.29 Typically i-CDI comprised of porous carbon cathode with net positive surface charge and net negatively charged carbon anode (Figure 3d).29 The i-CDI displays ion electrosorption during discharging whilst application of voltage causes desorption of the ions from the EDLs. Compared to conventional CDI system, a remarkable improvement in the desalination performance and ≈530 % increment in the electrode endurance was observed. It should be noted that the working potential window often employed in i-CDI operation is relatively small and it depends upon the potential of zero charge (EPZC) of both anode and cathode. Incorporation of either negatively or positively charged functional groups easily shifts the EPZC resulting in expansion of i-CDI working potential range. In i-CDI, the oxidation of anode surface by the formation of oxygen-containing functional groups is a boon, as it helps to shift the EPZC of the electrode which ultimately results in an enlargement of working potential range. Conversely, the continuous formation of the oxide layer has the full potential of increasing the anodic resistance that can lead to a decrease in ionic storage and a decline in the desalination performance capability.30
The salt ion removal capacity in CDI/mCDI system is somewhat limited while the energy consumption for desalination of high-salinity water exceeds than RO process,31 thus appears as a bottleneck towards desalination of sea or highly salinity water. This demands an investment of huge efforts to make desalination technologies more feasible in highly saline water. Desalination battery is made up of battery ionic electrodes for faradaic intercalation/deintercalation of ions of respective charges. Due to application of constant current for desalination and salination steps, the specific capacity of the electrode materials is defined in mAh g−1.12 Moving forward, by leveraging the pseudocapacitive property of metal oxide, another version of the CDI cell architecture utilizing metal oxides as electrode materials were developed. It is based on the desalination battery concept (Figure 3e),32 and the system is currently known as hybrid-CDI (or HCDI) cell architecture (Figure 3f), which consists of metal oxide as AEM and activated porous carbon as electrode.33 HCDI cell systems follow a strategy that involves replacement of one of the two electrodes by faradaic electrode. In addition to being able to avoid parasitic reactions, the use of faradaic electrodes under this strategy can also reduce the energy consumption in desalination. This is realized via the intercalation of ions through the chemical bonds between the crystal structures along with the absorption on the surface of the electrode material.34, 35
Generally, the electrodes employed for CDI are comprised of a few percentages of activated carbon (AC) while the rest is polymer binder which has no role in ion adsorption capacity of the material. In addition to this, the polymer decreases the ion removal efficiency of electrode material by blocking access to large pore volume part of the material.23 As aforementioned, a conventional CDI system involves desalination to produce fresh water followed by regeneration to form waste saline water. The utilization of fixed electrodes with limited access to AC somewhat limits the ion removal capacity of the CDI cell system. To alleviate this shortcoming, an interesting type of CDI architecture, based on the slurry-based electrodes used in electrochemical flow capacitors36 and semi-solid lithium-ion batteries,37, 38 utilizing carbon flow or carbon slurry flowing through the electrodes compartment was developed. Such CDI architecture is known as flow-electrode CDI (FCDI) as shown in Figure 3g–i.17, 33
FCDI offers two major benefits over conventional CDI cell architectures. The slurry-based electrode strategy involves continuous desalination of feed water stream while the discharge of the electrodes is performed separately downstream of the cell. On the other hand, conventional CDI performs the water desalination for finite time, after which regeneration of electrode is require for the next cycle. Secondly, the continuous input of activated fluidic carbon slurry in the electrode compartment offers an improved salt ion removal from the feed water, compared to static CDI cell architectures. In conclusion, all different modes of CDI offer unique advantages and disadvantages (see Table 1) when it comes to desalinating water. Traditional CDI (Figure 3a) is relatively slower but more robust and fouling-resistant, whereas pseudocapacitive HCDI showed faster desalination rate but required more costly electrode materials.
FB-CDI11 |
HCDI11 |
• Simple design, low energy consumption, and high salt removal capacity. |
• High salt removal capacity, energy efficient and scalable. |
• Low resistance to fouling and chemical contaminations. |
• Complex design, expensive, and low fouling resistant. |
|
|
• High salt removal efficiency. |
• Long-term operation stability and high ionic pollutant removal. |
• High feed pressure, and require large macropore electrodes.20 |
• Small working voltage window. |
|
|
mCDI13 |
|
• Low energy consumption, high salt removal capacity, longer electrode lifespan, and can operate in a wide range of flow rates. |
• Efficient energy conversion, low operating cost, and scalable. • Limited desalination capacity, and low efficiency than other energy storage systems. |
• Less efficient at high salinity, fouling, and expensive operation cost. |
|
|
|
FCDI11 |
|
• Excellent desalting efficiency at high salinity. |
|
• Saturation of carbon slurry may be controlled by optimizing flow rate and channel size. |
|
3 Electrode materials
The development of the CDI process has been greatly affected by the development of electrode materials, which are the core component of the entire process. A high-quality electrode material is a primary requisite for the high desalination performance of the CDI system. Generally, the electrode materials utilized in CDI system are made up of porous carbon materials such as AC,40 graphene,41 carbon nanotubes (CNTs),42 carbon fibers,43 carbon cloths,44 aerogel,45 etc. as described later. A huge amount of effort is being invested to design and develop novel electrode materials with improved ion separation performance. The key components for an ideal material that can be used as an electrode in CDI material area are: a) high specific surface area and the accessible pore size distribution to possess high salt electrosorption capacity, b) robust chemical stability under the working voltage in CDI, c) high conductivity and mobility of ions through the pores, and d) low cost.
To understand the materials requirements, below is the list of important parameters that decide the desalination performance capability of a material to be used as an electrode material for water desalination via CDI technology.
 (1)
(1)where, Q is the volumetric flow rate of effluent (mL min−1), Co and Ce are the initial and effluent salt concentrations (g L−1), melectrode is the mass of electrode material (g) that absorbs salt ion during water desalination.
 (2)
(2)where, Δt is the desalination operation time.
 (3)
(3)where, z is the number of charges on the ions, F is the faraday constant, and I is the current (Amperes).
 (4)
(4) (5)
(5)where, 1 and n denotes the first and nth cycles.
So far, varieties of electrode materials have been investigated. For simplicity, we have classified such types of electrode materials into three categories i.e. i) organic, ii) inorganic, and iii) polymer-based materials.
3.1 Organic electrode materials
3.1.1 Carbon-based materials
Carbon-based materials have been significantly investigated as electrode material in water desalination via CDI.47 Owing to the high specific surface area, low cost, robustness, and pore size distribution, ACs have been known as the most commonly used electrode material in CDI. For example, Liu et al. have explored the potential of commercially available ACs, derived from coal and wood, as electrode material in CDI.48 With a high specific surface area of 940 m2 g−1 and specific capacitance of 51.8 F g−1, the AC derived from coal displayed two-fold higher salt absorption capacity (SAC) than the wood-derived AC (specific surface area of 662 m2 g−1 and specific capacitance of 43.2 F g−1). Tan et al. demonstrated that the pore size and hetero atom content of the samples of disodium ethylenediamine tetraacetate pyrolyzed at 650, 750, and 850 °C varies significantly from each other, ultimately influence the electrosorption performance in water desalination.49 The sample pyrolyzed at 750 °C displayed the highest specific surface area of 976 m2 g−1 and SAC 8.02 mg g−1 at 25 mg L−1 concentration of NaCl solution which improves to 27.75 mg g−1 on increasing the concentration to 500 mg L−1. These observations infer that the specific surface and pyrolysis temperature of the material play a crucial role in improving the ion removal efficiency. In another study, Li et al. grafted the carbon nanotubes in the PVDF-derived porous carbons (PPC) via phase-inversion coupled with annealing method.50 Resulted PPC/CNT composite material showed an improved SAC of 15.1 mg g−1 in 500 mg L−1 NaCl solution compared to pristine PPC electrode SAC of 10.3 mg g−1. Furthermore, Arangadi et al. demonstrated a strategy where the desalination performance of the activated carbon (AC-FB) electrode was improved by introducing ion-dipole interaction sites in the structure.51 In this direction, a choline chloride urea, a hydrophilic deep eutectic solvent (DES), was merged with activated carbon to form AC-FB-DES. The prepared AC-FB-DES electrode displayed 85 % improved salt removal i.e. 28.60 mg g−1 in 600 mg L−1 salt solution at 1.2 V, compared to that of pristine AC-FB electrode.
3.1.2 COFs-derived carbon
For the application of porous carbon electrode materials in CDI, high porosity and high conductivity are primary requisites.52 However, the low SAC and co-ion expulsion during the charging process compromises the desalination performance of conventional porous carbon-based electrodes.53 To alleviate this shortcoming, faradaic materials consisting of redox-active species were utilized as electrode materials.54-57 Due to intrinsic redox-active property, such electrode materials were found cation-selective in nature complemented by less co-ion expulsion during charging. Covalent organic frameworks (COFs) are well-known 2D framework structure materials where the adjacent are stacked over each other via π-π interactions and generate well-defined pores/channels ideal for ion diffusion and EDL formation. Taking this into consideration, Li et al. introduced a redox-active DAAQ-TFP COF as a novel faradaic cathode material for the HCDI desalination process (Figure 4).58 The COF itself displayed a specific capacitance of 170.9 F g−1 and pseudocapacitive behavior. On the other hand, the nitrogen-doped porous carbon (NPC) derived from the carbonization of DAAQ-TFP COF in presence of ZnCl2 at 500, 700, and 900 °C under the nitrogen atmosphere, is utilized as the anode in the HCDI process. The NPC displayed SAC value of 22.8 mg g−1 and a desalination rate of 3.2 mg g−1 s−1 in a 500 ppm NaCl solution at 1.6 V.
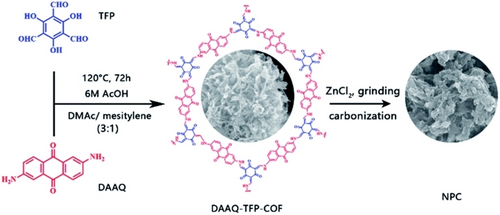
Synthesis of DAAQ-TFP COF and NPC. Reproduced with permission from Ref. [58]. Copyright 2019 American Chemical Society.
3.1.3 Carbon fiber
The structure of the carbon fiber consists of crystals that are more or less aligned parallel to the fibers along the axis. Generally, the carbon fibers display high SAC and high ASAR owing to the micropores and mesopores connected via a short diffusion pathway. For example, Liu et al. synthesized a porous carbon fiber (PCFs) derived from microphase-separated polymethyl methacrylate-block-polyacrylonitrile (PMMA-b-PAN) as an electrode material for CDI.59 In the structure of PCFs, the mesopores are well interconnected via micropores. Owing to the interconnected porous network structure, the PCFs facilitate a fast ion/electron transport pathway and displayed a SAC value of 30 mg g−1. In another interesting report, Lal et al., have reported electrospun porous carbon nanofibers (PCNFs) and TiO2 nanoparticles i.e. PCNFs/TiO2 composite decorated on carbon cloth and utilized as flexible electrodes for CDI.43 PCNFs/TiO2 displayed a specific surface area of 343 m2 g−1 with a pore volume content of 0.038 cm3 g−1. In addition, a specific capacitance of 110 F g−1 for PCNFs/TiO2 electrode was found higher compared to the pristine PCNFs electrode. In CDI performance, the PCNFs/TiO2 exhibits a large SAC of 204.8 mg g−1 in 500 ppm salt solution at 1.2 V and retains a salt absorption capacity of 96.4 % over 10 cycles.
3.1.4 Green carbon
Carbon materials derived from biomass feedstock are considered green materials due to their bio-based origin, low carbon footprint, low cost, and environmental friendliness. There are several reports available where biomass-derived ACs were utilized as an electrode material for water desalination.60 Lu et al. prepared biomass-derived porous AC nanoflakes (PCNs) from pyrolysis of a mixture of xylose and KHCO3 mixed in different mass ratios and heated at 450 °C, and finally annealed at 700 °C under inert atmosphere.61 The PCNS sample displayed a SAC of 16.29 mg g−1 for 1 g L−1 NaCl solution at 1.2 V and maintains high salt removal efficiency up to 25 cycles. Maniscalco et al. derived porous carbon from almond shell pyrolyzed at 800–1000 °C and CO2 activation.62 The carbon material obtained at 900 °C exhibits a maximum SAC value of 19.2 mg g−1. Hai et al. derived porous carbon from date seeds by performing pyrolysis at 900 °C in presence of KOH.63 The derived AC exhibit the specific capacitance of 400 F g−1 and SAC of 22.5 mg g−1 at 1.2 V. Liu et al. prepared utltraporous carbon nanosheets derived from carbonization of lotus leaf at 500 °C.64 The prepared electrode material displayed a high surface area of 4482 m2 g−1 and a specific capacitance of 225 F g−1 in 1 M NaCl solution. In desalination performance, the prepared material exhibits a SAC of 13.5 mg g−1 at 1.2 V which significantly improves to 65.0 mg g−1 on raising the potential to 1.6 V. In a recent report, Zhang et al. prepared a super-activated carbon (AC-P-CO2) from pyrolysis of H3PO4 treated coconut shell at 865 °C followed by CO2 activation.65 With surface area of 2617 m2 g−1 and high pore volume of 2.3 cm3 g−1, AC-P-CO2 exhibit SAC value of 67.6 mg g−1 after 10 cycles in 500 mg L−1 NaCl solution at 1.2 V and charge efficiency of 76.5 %. Govindan et al. utilized a leaf base of Phoenix dactylifera, usually known as palm tree waste, as a source of preparing AC electrodes.66 In this direction, the powdered leaf waste was first carbonized at 400 °C for 4 h followed by treatment with KOH and annealing at 950 °C for 4 h to afford AC. The AC material was then modified by hydrothermal method by adding α-MnO2 to obtain α-MnO2/f-AC composite electrode. α-MnO2/f-AC composite electrode possess higher specific capacitance of 388 F g−1 and SABET of 753 m2 g−1, suggesting the porous nature of the electrode. In water desalination performance, the prepared α-MnO2/f-AC composite electrode displayed a SAC of 17.8 mg g−1 in a 600 mg L−1 feed solution at 1.2 V.
3.1.5 Graphene-based materials
Graphene is a two-dimensional sheet material with 1 atom thickness. Owing to the conjugated structure, graphene exhibits excellent ionic conductivity and thus has found wide application in the CDI desalination process.41 Cuong et al. investigated the effect of the incorporation of nickel hexacyanoferrate (NiHCF) in the reduced graphene oxide (rGO) bulk utilized as a cathode material (NiHCF@rGO) for desalination process in asymmetric CDI.67 The NiHCF@rGO nanocomposite material, prepared by pseudocapacitive intercalation of NiHCF in the rGO, displayed a specific capacitance of 158.2 F g−1 and SAC of 80.2 mg g−1 for 4 g L−1 NaCl solution at 1.2 V, and charge efficiency of 86.6 %. The SAC value showed by NiHCF@rGO was improved compared to pristine NiHCF CDI performance (SAC of 62.8 mg g−1), owing to the high conductivity and charge transfer by incorporation of rGO with NiHCF. Xu et al. synthesized graphene oxide/CNTs sponge (GNS) by freeze-drying treatment of graphene oxide and CNTs mixed solution followed by annealing under inert nitrogen atmosphere.68 The GNS loaded with 20 wt % of CNTs exhibits a surface area of 498 m2 g−1 and a specific capacitance of 203.48 F g−1. In the application as a CDI electrode, GNS exhibits SAC of 18.7 mg g−1.
There are several reports available where conductivity of the graphene-based material has been improved by doping with chemicals or metals. For example, Bharath et al. prepared the Mn3O4/rGO composite by grafting manganese oxide nanowires on the rGO via hydrothermal process.69 The Mn3O4/rGO composite material exhibited a specific capacitance of 437 F g−1. In desalination application, it showed a SAC of 34.5 mg g−1 in 1 g L−1 NaCl solution at 1.2 V and ASAR of 1.15 mg g−1 min−1. In another study, a FGC-ZnO composite made up of graphene, CNT, and ZnO nanoparticles (5 % and 10 % loading) was used as an electrode material.70 The FGC-ZnO-10 % material exhibits a high specific capacitance of 280 F g−1 and SAC of 28.6 mg g−1 in 600 mg g−1 NaCl solution at 1.2 V, compared to FGC-ZnO-5 %. Ma et al. have loaded mesoporous iron phosphate in rGO to prepare mesoporous FePO4 nanosphere@graphene (FePO4@rGO) using a one-step hydrothermal method and applied it as an electrode material in a hybrid CDI desalination cell system.71 In desalination experiments, FePO4@rGO exhibits an excellent SAC of 53.71–85.94 mg g−1 accompanied by a decrease in energy consumption by 25 %, owing to improved electrical conductivity and ion diffusion. Xi et al. demonstrated a strategy where they prepared a CuAl-LDO/rGO anode comprised of vertical-aligned CuAl layered double oxide grown on rGO for application in a hybrid CDI desalination process.72 Owing to the graphene conductivity and fast diffusion of chloride ion the CuAl-LDO/rGO material displayed a SAC of 64 mg g−1 in 1 g L−1 NaCl solution at 1.2 V which negligibly decrease to 58 mg g−1 retaining salt ion absorption capacity of 90 % after 20 cycles. This decrease in electrosorption performance was attributed to the aggregation of CuAl-LDO/rGO particles and the formation of Cu(OH,Cl)2.2H2O.
3.2 Inorganic electrode materials
3.2.1 MOFs-based carbon
Metal organic frameworks (MOFs) are well known for their high porosity, crystallinity, ordered structure, functionalization, pre- and post-synthetic modifications, and thus, have potentially found wide applications in optoelectronics, sensing, catalysis, separation, adsorption, etc.73 However, MOFs usually suffer from low chemical stability and poor electrical conductivity which limits their usefulness. The conductivity of the MOFs can be enhanced either by doping i.e addition of electrically conductive material or by direct pyrolysis of MOFs at higher temperatures.74 For example, Liu et al. synthesized a porous carbon polyhedral (PCP) by carbonization of ZIF-8 at 800–1200 °C.56 Compared to other PCPs, the PCP-1200 exhibited a high specific capacitance value of 275.69 F g−1 and SAC of 13.8 mg g−1 in 500 mg L−1 at 1.2 V while maintaining consistent desalination over 30 cycles. Song et al. prepared nitrogen-rich hollow bowl-like carbon (HBC) derived from pyrolysis of bowl-like B-ZIF-8 at 800 °C.75 In CDI, the HBC sample exhibited a specific capacitance of 210.7 F g−1 and SAC of 28.06 mg g−1 for 500 mg L−1 NaCl solution while maintaining salt absorption capacity of 90 % after 20 rounds. The high CDI performance of HBC materials was attributed to the improved conductivity due to nitrogen doping and the short ion diffusion pathway provided by nano-scale wall thickness, regular microporous structure, abundant ion adsorption active sites, and interconnected channels facilitating the fast transfer of salt ions. In another study, Li et al. synthesized a hierarchical porous carbon (MMCK) from carbonization of Mg-MOF-74 (Figure 5)76 at 650 °C followed by KOH activation.77 The carbonized MMCK sample displayed a specific capacitance of 240 F g−1 and SAC of 16.82 mg g−1 for 500 mg L−1 NaCl solution. The electrosorption capacity efficiency retains to 96.7 % after 20 cycles.

Single crystal structure of Mg-MOF-74 formed by the reaction of the DOT linker with Mg(NO3)2.6H2O. Reproduced with permission from Ref. [76]. Copyright 2009 Proceedings of Natural Academic Sciences.
3.2.2 Metal-oxide-based electrode materials
Owing to their intrinsic conductivity, metal-oxide-based materials have emerged as promising electrode materials for the CDI desalination process. Sun et al. prepared an oxygen-doped molybdenum disulfide electrode material via the hydrothermal method.78 The oxygen-incorporated MoS2 nanosheets were utilized in hybrid CDI cell system to investigate the effect of oxygen-doped MoS2 nanosheets on the electrosorption performance. The as-synthesized material exhibits SAC of 28.85 mg g−1 in 500 mg L−1 at 0.8 V, which is 5.5 folds higher than the pristine MoS2 nanosheets. Bentalib et al. investigated the electrosorption performance of electrode material made up of iron phosphate, carbon black, and Nafion.79 The prepared electrode efficiently removes sodium ions up to 7.6 wt %. In the hybrid CDI desalination process, the material displayed the SAC value of 82 mg g−1 and current efficiency of 90 % in 10 mM NaCl solution at 1.2 V. Wen et al. grew a vertically aligned Co(OH)2 nanosheets on graphite sheet via hydrothermal strategy.80 Obtained Co(OH)2@GP material showed a specific surface area of 207.62 m2 g−1. In electrosorption performance, the Co(OH)2@GP showed the SAC of 48.21 mg g−1 and specific capacitance of 411 F g−1 in 300 mg L−1 in NaCl solution at 1.2 V, due to its high surface area, high conductivity, and accessible pore network. Liu et al. prepared sodium-incorporated cobalt oxide i.e. NaxCoO2 with varying amounts of sodium metal (x=0.2–1.6) in the structure.81 The interlayer space between the two adjacent CoO6 octahedral layers provides enough space for intercalation and de-intercalation of sodium ions. This led to the high conductivity of the obtained material. In the hybrid CDI process, the prepared material displayed SAC of 63 mg g−1 at 1.4 V, and specific capacitance of 198 F g−1, and charge efficiency of 97 %. Later, Zhou et al. doped the NaxCoO2 with Zn to boost the conductivity of the material by creating electronic holes utilizing a strategy of partial substitution of Co ions with Zn ions.82 In CDI, the as-synthesized materials reached the SAC value of 125.3 mg g−1 in 1 g L−1 NaCl solution at 1.4 V, and capacity retention to 88.6–98.3 % over 50 cycles.
3.2.3 MXenes-based materials
MXenes are two-dimensional layered structure materials that consist of a few atom-thick layers of transition metal carbide, nitrides, or carbonitrides.83 Due to the presence of hydroxyl- or oxygen-terminated functional groups at the surface, MXenes are often hydrophilic in nature. MXenes nanosheet structure easily allows reversible intercalation/deintercalation of ions.84 MXenes are usually synthesized by top-down selective etching approach with no deterioration in the chemical properties with scalability. Generally, the bonding interactions between the two adjacent MXene layers are weak. As a result, they can be easily intercalated or doped with guest molecules such as urea, hydrazine, etc. Due to the conductive layered structure and hydrophilic surface, MXenes have been widely explored in the fields such as energy storage devices, for example, batteries and supercapacitors, sensors, photocatalysts, water purification, etc. The utilization of MXenes as electrode material in the CDI desalination process is associated with two major advantages. First, as seen in the traditional carbon electrodes the salt ions are stored in the EDL region generated at the electrode surface. On the other hand, the MXenes-based electrodes easily store the salt ions within the layers via intercalation or faradaic process. Secondly, MXenes layered structure selectively absorbs either cation or anion which further aid to prevent co-ion expulsion effect resulting in a higher charge efficiency of the electrode in the CDI desalination process. In a report, Ai et al. prepared a self-healing hydrogel (MNH) composite from MXene Ti3C2Tx nanosheets and poly(vinyl alcohol)-based network using diol-borate ester cross-linkage.85 The resulting material comprised channels accessible for ion diffusion and resistance to MXene accumulation. Electrosorption studies showed the SAC value of 51 mg g−1 in 1 g L−1 NaCl solution at 1 V, which is attributed to the high conductivity of the structure. Liang et el. prepared a hydrophilic Ti3C2Tx/Ag composite involving uniform distribution of silver nanoparticles in the layered structure of Ti3C2Tx via facile oxidation-reduction method.86 The obtained material was found highly conductive due to low charge transfer resistance. Ti3C2Tx/Ag composite displayed a SAC value of 135 mg g−1 in 10 mM NaCl solution and low energy consumption of 0.42 kWh kg−1.
MXene nanosheets are highly susceptible to agglomeration of MXenes and self-restacking due to strong interlayers van der Waals interactions.87, 88 Consequently, MXenes are often observed to exhibit low chemical stability in natural saline water which causes downgrade in the desalination performance and recyclability of MXenes in CDI application.89 Wang et al. loaded iron oxide nanoparticles inside the layers structure of Ti3C2Tx to form Fe3O4@Ti3C2Tx heterostructure.90 Incorporation of iron oxide nanoparticles was found to significantly improve the charge transfer and prevent oxidation as well as the agglomeration of MXene within the structure. Taking advantage of pseudocapacitive behavior of Fe3O4 and sodium storage ability of MXene, the Fe3O4@Ti3C2Tx as cathode in HCDI process displayed SAC value of 44 mg g−1 in 500 mg L−1 at 1.2 V maintained even after 40 cycles. In an interesting report, Zhang et al. prepared (Ti3C2Tx@DAAQ-TFP) an MXene-organic 2D heterostructure by treating MXene-NH2 with DAAQ followed by TFP via Schiff-base condensation reaction (Figure 6).91 The prepared MXene@COF benefits from the excellent ionic conductivity of MXene accompanied with high specific surface area, integrated redox-active anthraquinone unit and hierarchical porous structure of COF. The COF layer present on the surface prevents the MXene nanosheets realignment to protect the structure from getting oxidized during desalination/regeneration process while the inner MXene nanosheets enhance the conductivity of the MXene@COF via phenomenon of reversible transport of ions and electron. In addition to this, the redox-active DAAQ unit present in the MXene@COF skeleton delivers the fast transport of sodium ion which further enhances the absorption of faradaic sodium ions during the desalination process. In CDI desalination process performed for oxygen-containing saline water, the MXene@COF displayed the SAC value of 53.1 mg g−1 while maintaining cyclic stability over 100 cycles.
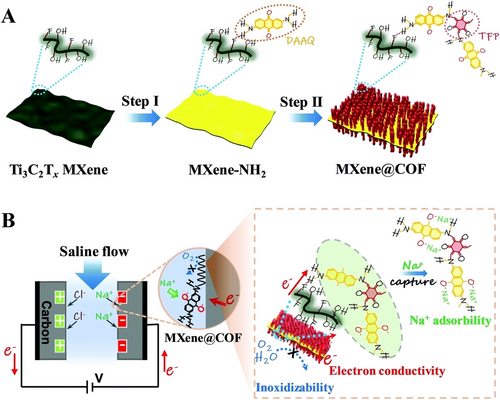
Schematic illustration for heterointerface optimization in the MXene@COF heterostructure and its promotion of the CDI process. Reproduced with permission from Ref. [91]. Copyright 2022 The Royal Society of Chemistry.
3.2.4 Prussian blue-based materials
Prussian blue (PB) and its derivative materials are large three-dimensional framework materials that consist of widely distributed interstitial sites/channels available for ion diffusion.92 PB-based materials are usually represented as A2−xMa[Mb(CN)6]1−y.nH2O where A represents alkali ions, and Ma and Mb stand for transition metal ions such as Mn, Fe, Co, Ni, Cu, Zn, etc. Interestingly, the [Mb(CN)6]4− unit tunability and high structural stability during intercalation/deintercalation process suggest that PB materials are potential candidates that can be utilized as electrode material in the CDI desalination process.93, 94 In literature, there are reports where PB-based materials were employed to boost the electrochemical performance of the electrode in energy storage devices such as batteries92 and supercapacitors.95 Despite such advantageous features, PB-based materials suffer from the drawback of charge polarization and low ionic conductivity.96, 97 To address this drawback, Wang et al. demonstrated that integration of rGO98 or CNTs99 with cobalt hexacyanoferrate PB derivative delivers the material possessing high ionic conductivity and energy density.100
Inspired by this, Zhang et al. prepared a homogeneous particle size flexible binder-free PB-based electrode (PBAs/CC), PB analogues of Mn, Co, and Cu grown on flexible carbon cloth support.101 The Mn-PB, Co-PB, and Cu-PB displayed the SAC value of 7.96, 14.47, and 8.56 mg g−1 respectively at 1.0 V, compared to pristine CC (SAC of 7.19 mg g−1). In addition, the low energy consumption of 0.389 kWh m−3 shown by Co-PB for desalination compared to pristine CC (0.778 kWh m−3) was also noted. These observations highlight the benefits of combining chemical stability and fast ion diffusion properties of PB with conductive flexible carbon cloth to afford the electrode materials possessing improved electrochemical performance for the CDI desalination process.
3.3 Polymer-based electrode materials
These are conducting polymers including polypyrrole (Ppy), polyaniline (PANI), polythiophene (PTP), etc., which are intrinsically conducting in nature. Compared to conventional polymers, they are easily processable mainly by dispersion. However, they are organic materials and conducting in nature but do not exhibit mechanical properties like other conventional polymers. Intrinsic conductivity in such polymers originates from the movement of electrons present in a one-dimensional electronic band formed by the conjugated p-orbitals.
3.3.1 Synthetic polymers
Owing to its high conductivity, chemical stability, and redox properties, Ppy has been extensively studied among conducting synthetic polymers. However, poor cyclic stability is among one of its demerits that limit its usefulness. On the other hand, PANI has been known for its high-temperature tolerance, chemical stability, and excellent electrical conductivity. Poor mechanical stability, poor solubility in different solvents, and strong gelation tendency are some of the disadvantages associated with PANI. In contrast, PANI in tubular form offers more space for electrolyte storage and thus can facilitate fast ion diffusion. Taking this into consideration, Nie et al. prepared PANI/AC composite by casting a slurry consisting of PANI dispersed in AC and 10 % of polytetrafluoroethylene (PTFE).102 In inverted hybrid CDI cell system, the PANI/AC attained charge capacitance of 289 F g−1 with SAC of 30.5 mg g−1 compared to a pristine AC electrode (SAC of 11 mg g−1). On the other hand, Shi et al. developed a strategy for grafting uniform-sized Prussian blue nanocrystals on the tubular PANI, which ultimately improved the conductivity of the material and pseudocapacitance performance with SAC of 133.3 mg g−1 including the longevity of the electrode up to 250 cycles.103 In another work, Wang et al. demonstrated the effect of mesopores on the desalination capacity of the material in CDI.104 In this regard, they prepared an N-doped porous carbon (NPC) by pyrolysis of PAN using nano-silica and ZnCl2 as pore-making reagents. NPC displayed a specific surface area of 1903 m2 g−1 with SAC of 19.61 mg g−1 and a specific capacitance of 194.75 F g−1 for 500 mg L−1 NaCl solution at 1.2 V.
3.3.2 Bio-based polymers
Chitin, chitosan, and cyclodextrin are some examples of known bio-based polymers used as electrode material in CDI applications. For example, Zhang et al. fabricated a bio-derived chitosan-based carbon electrode (CCS/GCNS) via incorporation of g-C3N4 nanosheets in the 3D chitosan-based carbon material using a hydrothermal-activation strategy (Figure 7).105 The incorporation of g-C3N4 nanosheets was found to enhance the conductivity, porosity, and wettability properties of the CCS/GCNS. The prepared CCS/GCNS electrode displayed a surface area of 2529.3 m2 g−1 and nitrogen content of 9.8 %. Furthermore, a specific capacitance of 312.8 F g−1 and SAC of 32.8 mg g−1 for 10 mM NaCl solution at 1.2 V while maintaining the salt ions removal efficiency to 95 % even after 50 cycles was observed. An improved conductivity of CCS/GCNS material suggests the potential of metal-free bio-based green polymers in the CDI desalination process. In another study, porous biochar (HC-800) derived from thermal deposition and chemical activation of chitin using KOH was reported.106 The KOH-activated biochar displayed an improved surface area of 833 m2 g−1 and charge capacitance of 122.33 F g−1 compared to the pristine biochar (surface area 126 m2 g−1 and charge capacitance of 42.87 F g−1). HC-800 displayed SAC value of 11.52 mg g−1 and decent recyclability compared to the pristine biochar analogue. In another report, Zhang et al. synthesized a nitrogen-doped porous carbon (NPPC) from polyporphyrin using KOH-activation performed at 700–900 °C under an inert nitrogen atmosphere.107 In addition to the high stability and conductivity, the NPPC exhibits a surface area of 2979.3 m2 g−1 and a pore volume of 2.22 cm3 g−1 with SAC value of 35.7 mg g−1 in 10 mM of NaCl solution at 1.2 V while maintaining electrosorption capacity efficiency to 95 % over 50 cycles, and specific capacitance of 328.7 F g−1. Ma et al. synthesized a composite of sulfobutylether β-cyclodextrin and carbon nanotubes.108 The modified CNT electrode displayed a capacitance of 60.9 F g−1 and high hydrophilicity (water contact angle, WCA 65.5°) compared to the pristine CNT electrode (capacitance 51.7 F g−1 and WCA 103.6°). This could be attributed to the synergistic effect of the presence of hydroxyl and sulfonic groups on the surface resulting in efficient ion transfer. For a modified CNT electrode, an improved value of SAC of 6.37 mg g−1 and CE of 36.9 % suggest the cation selectivity over the pristine CNT (SAC of 4.17 mg g−1, and CE of 25.8 %).
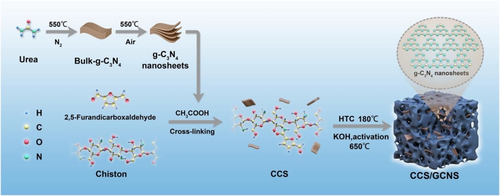
Synthesis of CCS-GCNS. Reproduced with permission from Ref. [105]. Copyright 2022 Elsevier.
4 Membrane capacitive deionization (mCDI)
Generally, in CDI there is a constant release of co-ions into the feed water stream via diffusion which is an adverse phenomenon that deteriorates the performance of materials in water desalination process.13, 109 By contrast, the co-ion expulsion effect can be abbreviated by accommodating the ion exchange membranes (IEMs) between the electrodes. A curtailment in the extrication of co-ion in the stream has been considered the main factor responsible related to the enhanced performance of the mCDI. Employing placing IEMs next to the electrode, acts as a barrier for the absorbed ions and restricts them from leaving the electrode surface. When voltage is applied between the electrodes, the counterions are absorbed in the micropores whilst the presence of selective IEMs positioned in front of the electrodes assists the co-ion accumulation in the macropore region of the electrodes rather than their release into feed water stream. Escalation in the concentration of co-ion in the macropores compared to the concentration in the feed water leads to the buildup of excess charge in the macropores. To neutralize the charge, IEMs allow more counterions to pass through into the electrode area, ultimately enhancing the salt ion removal potential of the material with each cycle of mCDI.11 In addition, during desorption IEMs allows the complete removal of ions and regenerate the electrode for the next desalination cycle. In short, a symbiotic contribution of both micro- and macropores of the electrodes related to salt ion removal capacity of the mCDI system eventually improves the water desalination performance compared to CDI. In CDI, an anode is often susceptible to structural degradation by oxidation of carbon electrode which drastically deteriorates the desalination performance. On the contrary, mCDI offers a longer lifetime of electrode materials. IEMs placed in front of the electrodes act as a barrier for the direct contact of the electrode with feed water. As a result, IEMs avoids detrimental structural degradation reaction at the anode which often hampers the water desalination performance.
Ion exchange membranes (IEMs) used in mCDI act as a co-ion barrier and help to minimize the co-ion expulsion effect and also avoid the co-ions carrying the parasitic current which are responsible to lower the charge efficiency and electrosorption performance of a material in water desalination process. There are several materials reported in the literature utilized as IEMs for mCDI application. The primary requirements for a material to be applied as IEMs in mCDI are high conductivity, high wettability, short ion-diffusion pathway, and high surface area. Commercially available IEMs suffer from the drawbacks of high thickness, high ionic resistance, hindered ion-diffusion pathway, and poor ion-exchange capacity. However, the application of nanomaterials such as MOFs, MXenes, graphene, etc. as IEMs for mCDI are less studied. Nevertheless, the high conductivity, chemical stability, and mechanical properties associated with such materials make them a potential candidate for IEMs. IEMs are of two types: cation exchange membranes (CEM) which allow cations to pass through, and anion exchange membrane (AEM) which allows anions to permeate through. IEMs are typically made up of polymers embedded with charge carriers grafted by surface modification via chemical treatment.110 CEMs are often comprised of quaternary ammonium ions (NH4+) whereas AEMs consist of sulfonic groups (SO3−) or phosphate ions (PO3−). The presence of a functional group in the structure of the material enhances the charge carrier potential of the membrane by attracting the opposite charge ions and restricts the diffusion of ions of the same charge (co-ions).
To evaluate the desalination performance of the IEMs, there are certain membrane parameters described in the literature such as area resistance (Ra), water uptake (WU), linear swelling ratio (LSAR), ion-exchange capacity (IEC), and permselectivity.111 In this part, we have briefly discussed the abovementioned parameters followed by the ion exchange materials used in mCDI desalination process.
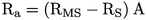 (6)
(6)where, RMS is the resistance of the membrane dipped in 1 M NaCl solution, RS is the internal resistance of the cell, and A is the exposed area of the membrane.
 (7)
(7)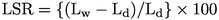 (8)
(8)where, Lw and Ld are the lengths of wet and dry membranes, respectively.
Permselectivity in IEMs describes the degree to transfer the ions of one charge pass through the membrane while the co-ions are retained.112 A unit value of permselectivity means the transports of the ions are completely blocked by the IEM. A lower value of the permselectivity suggests the absorption of co-ions. For high salt ions removal and charge efficiency in mCDI, a robust, high-performance, and cost-effective ion-exchange material is requisite to corroborate the minimization of co-ion expulsion effect and longevity of the electrode in long-run industrial mCDI applications. In this line, low area resistance, high permselectivity, high WU, low LSR, and high IEC are some of the features to be present in the materials utilized for mCDI.
5 Membrane materials
In this part of the review, we have divided the discussion related to the variety of materials that have been developed as IEMs for mCDI.
5.1 Organic membrane materials
5.1.1 Graphene-oxide-based IEMs materials
As per the strategy of preparation of conductive IEMs, highly conductive rGO is often blended with the polymers to fabricate conductive polymeric membranes for water desalination. For example, Zhang et al. have synthesized a conductive AEM composite via dispersion of rGO/PANI composite in the polyvinylendene (PVDF) polymer matrix using the dry phase inversion method.113 By means of dispersing rGO in polymer matrix, they were able to load up to 50 % of PANI inside the polymer matrix. This has ultimately increased the wettability of the as-synthesized AEM as a result of which it displayed high conductivity and SAC value of 1.56 mg g−1 and salt removal efficiency of >90 % in mCDI desalination process. Qian et al. have developed a strategy to prepare a high-performance CEM via uniform coating of sulfonated graphene on carbon nanofibers using a dip-coating approach.114, 115 The presence of sulfonated terminal functional groups at the surface made the membrane surface hydrophilic. Compared to the pristine CNFs, the sulfonated GO exhibits two folds higher SAC of 9.5 mg g−1 and charge efficiency of 43 %.
5.1.2 COFs-based IEMs materials
In comparison to polymers, COFs offer ordered structure, porosity, chemical robustness, thermal stability, and crystallinity. Generally, the COFs are non-ionic.116, 117 However, the conductive COFs can be easily designed and synthesized through the incorporation of ionic organic linker in the COFs skeleton.116 McNair et al. have demonstrated a strategy in which guanidinium-based ionic covalent organic nanosheets (iCONs) were loaded in the matrix of quarternized polybenzimidazole (QPBI) polymer to prepare iCON@QPBI AEM for mCDI (Figure 8).118 The iCON@QPBI AEM exhibits a SAC value of 15.6 mg g−1 (50 % higher than pristine QPBI) and a charge efficiency of 90 % (20 % higher than pristine QPBI). The presence of additional quarternized ammonium groups improves the hydrophilicity of the AEM. Moreover, iCONs loaded inside the QPBI matrix provide an ordered channels structure facilitating fast ion conducting pathway for salt ions removal in mCDI desalination process. In another study, Li et al. demonstrated the facile strategy to prepare the N,B-doped porous carbon spheres (PCS) for mCDI.119 The PCS were synthesized via carbonization of TAB-FPBA COF followed by annealing under an inert nitrogen atmosphere. Owing to the dual doping and hierarchical pore distribution of porous carbon spheres, the as-synthesized material applied SAC value of 18.5 mg g−1.
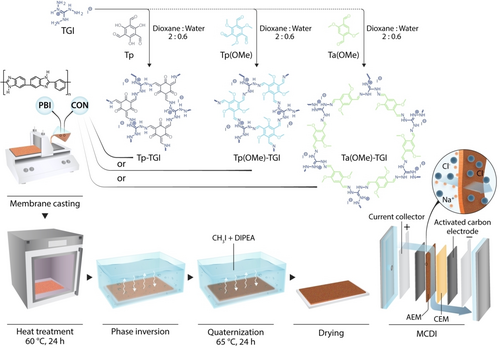
Schematic of the preparation of both the ionic covalent organic nanosheet (iCON) materials and nanocomposite membranes used for membrane capacitive deionization. Reproduced with permission from Ref. [118]. Copyright 2022 Elsevier.
5.2 Inorganic materials
5.2.1 Metal-oxide-based IEMs materials
Owing to the intrinsic pseudocapacitive behavior and material diversities, the utilization of metal-oxide-based materials as an electrode in CDI has resulted in improved ion removal capacity by ion intercalation and additional redox reactions. Generally, the charge efficiency and SAC performance of the material are hampered by the co-ion expulsion and parasitic reaction that occurred during charging. To address this issue, Wu et al. attempted to prepare AC electrode coated with a thin layer of ion-exchange polymer for mCDI (Figure 9).120 In this direction, they have employed montmorillonite (MT, Al2O9Si3) as an anion-exchange material and hydrotalcite (HT, Mg6Al2(CO3)(OH)16.4H2O) as a cation-exchange material for the preparation of electrode. A charge efficiency of 90.5 % and SAC value of 15.8 mg g−1, compared to the pristine sample (CE of 55 % and SAC of 10.2 mg g−1), suggest the potential of montmorillonite and hydrotalcite as ion exchange materials for the fabrication of high-performance charge efficient electrodes for mCDI. In another report, Liu et al. fabricated a binder-free polyoxometallate-based mixed capacitive-deionization electrode for seawater mCDI desalination.121 The electrode was fabricated via electro-deposition of SiW12O404−/PANI nanoparticles (size 100–120 nm) on the exfoliated graphite bulk. The prepared material showed a specific capacitance of 352 F g−1 and SAC value of 23.1 mg g−1 in 500 mg L−1 NaCl solution at 1.2 V while maintaining consistent salt ion removal efficiency even after 30 cycles. An excellent desalination performance of polyoxometallate-based electrodes was attributed to the synergistic effect between SiW12O404− and PANI. Feng et al. demonstrated the improvement in the desalination performance of free-standing CNT-based membranes prepared by deposition of TiO2 on CNTs via the atomic layer deposition (ALD) method.122 The TiO2-deposited membranes displayed a two-fold higher SAC value of 3.33 mg g−1 compared to pristine CNT electrodes (SAC of 1.35 mg g−1). This is attributed to the increased hydrophilic character of the electrode by TiO2 deposition facilitating enhanced diffusion of the ion into the pores of the electrode. El-Deen et al. have demonstrated an efficient method to fabricate TiO2 nanorod-intercalated rGO as electrode material for mCDI.123 The nanocomposites at 20 wt % TiO2 loading showed an improved SAC value of 9.1 mg g−1 and a specific capacitance of 443 F g−1, a nine-fold higher than to pristine rGO electrode.
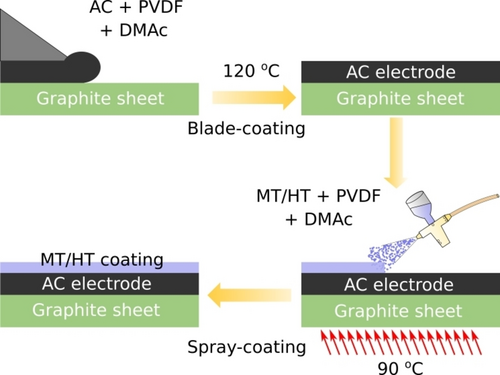
Schematic diagram of the preparation of ion-exchange inorganics-coated electrode. Reproduced with permission from Ref. [120]. Copyright 2021 American Chemical Society.
5.2.2 MOFs-based materials
TiO2 has been identified as an effective pseudocapacitive material due to its chemical stability, non-toxicity, high capacity, and abundant nature. However, it often suffers from poor conductivity and low ion diffusion rates. In this aspect, several strategies such as the incorporation of nanoparticles of TiO2 in AC, CNTs, CNFs, rGO, etc. have been reported. Despite of this, due to poor internal bonding between TiO2 and carbon structure, such materials often displayed a low SAC in water desalination applications. To alleviate the issue, Ding et al. have demonstrated a strategy where they pyrolyzed the Ti-based MOF at different temperatures and investigated the electrosorption performance of three composites for mCDI.124 Pyrolysis of Ti-MOF (MIL-25) at 600, 800, and 1000 °C under argon atmosphere delivered TiO2@PC composites comprised of TiO2 polymorphs (anatase, rutile, mixed). The specific surface area and graphitic degree of carbon content in the composite were found to increase with an increase in the annealing temperature. However, the TiO2@PC-600 exhibits a higher SAC value of 46.7 mg g−1 compared to TiO2@PC-1000 (SAC of 34.4 mg g−1). An improved desalination performance of TiO2@PC-600 as an electrode in mCDI is attributed to the high pseudocapacitance of anatase TiO2-polymorph while porous carbon contributed via providing high conductivity and fast ion diffusion pathway.
5.3 Bio-waste-derived IEMs materials
Synthesis of conventional porous carbon often requires harsh conditions which inevitably makes the process costly, time-consuming, and less eco-friendly.125 According to recent reports, carbonization of biomass wastes was performed to deliver the engineered biochar-mineral composites.126 Activated porous biochar was often prepared by chemical e.g. KOH/ZnCl2 or CO2/Steam treatments.127 However, the formation of large-scale disordered micropores in the prepared biochar was found to limit ion transfer to the pores. Later, a two steps strategy i.e. pyrolysis followed by steam activation was adopted for the preparation of biochar.128 To develop a time-efficient and simple synthesis approach for biochar, Tang et al. prepared a porous carbon from microwave treatment of sugarcane bagasse followed by the activation by CO2 or N2 at different flow rates.129 The prepared material possesses a specific surface area of 1019 m2 g−1 and displayed a specific capacitance and SAC value of 208 F g−1 and 28.9 mg g−1, respectively, in 5 mM NaCl solution at 1.2 V.
Figure 10 displays a SAC vs ASAR comparison of the various electrode materials used for water desalination. However, due to inconsistency in the various water deionization performance parameters while reporting results limit us to present a fair comparison (see discussion in section 9).
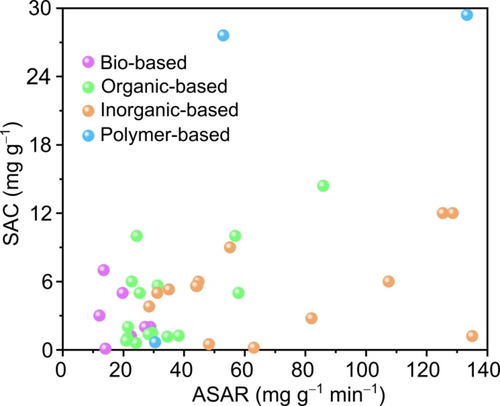
Comparison of the performance of various types of electrode materials explored in CDI/mCDI technique.10
6 CDI/mCDI vs. other membrane-based water desalination technologies
Many evaluations have been published comparing CDI/mCDI with RO and ED for water desalination based on energy and economic calculations, but no consensus has been achieved.130, 131 In general, mCDI has significantly lower energy consumption than traditional membrane-based desalination technologies, such as RO, NF, and UF132 by avoiding the use of high-pressure pumps required in RO and the external power sources in ED. The presence of IEMs in mCDI reduces organic and inorganic fouling because of periodic reversal polarity electrical field. Although both RO and thermal desalination methods are commonly used for saltwater desalination, RO accounts for 86 % of global brackish water desalination capacity.133 According to Sharan et al. findings, in comparison to RO, the cost-effectiveness of CDI relies on the feed concentration but is better for salt concentrations less than a) 70±15 mM (surface brine charge), b) 65±13 mM (deep well injection), and c) 43±10 mM (evaporation pond).134 Regardless of salt concentrations, the CDI is not cost-effective for zero liquid discharge when compared to RO.135 Furthermore, CDI has demonstrated its ability to have a lower carbon footprint than the current RO system for brackish water desalination. However, some drawbacks restrict CDI′s economic utility for saline water desalination: 1) CDI is frequently performed in batch mode, which means that water is only desalinated during the charge period. The use of flow electrodes CDI cell design for continuous mode operation complicates the architecture and raises the overall expense of the operation,39 2) compared to an industrial RO system, a steady charging/discharging current and flow rate usually yield poor water recovery in CDI.136 A low recovery ratio results in more discarded brine, which can cause serious environmental issues and necessitates appropriate disposal methods, and 3) CDI discharge cycle is a daunting task that consumes nearly 50 % of the overall cycle time, reducing process output.137
7 Miscellaneous CDI parameters
The electrosorption performance of electrode material also depends on some other important factors such as composition of the feed water, material properties, and experimental parameters used in CDI. In this section, the various factors that control the electrosorption performance of the materials have been discussed in brief.
7.1 Synthesis conditions
Interparticle porosity and approach utilized to prepare electrodes can influence the desalination performance in CDI. In this aspect, Barcelos et al. performed a systematic investigation related to the desalination performance of the electrode prepared via two methods: free-standing (FS) and blade-casting (DB) method.138 Although, the gravimetric SAC values are the same i.e. 14.7 and 16.9 mg g−1, the volumetric salt adsorption capacities suggest that the electrode prepared by DB is better than FS method.
The thickness, wettability, and interparticle porosity of the material fabricated by different methods could result in varying textural and electrical properties. Better performance of DB electrode originating from the fast mass transfer through the less thick membrane is possible via large interparticle voids created during the FS casting approach. In a report, Cerón et al. developed a strategy where an introduction of minor modification in the synthesis procedure resulted in hierarchical carbon aerogel monolith (HCAM) being made selective for adsorption of calcium or sodium ions.139 For example, a resorcinol-formaldehyde organic aerogel was carbonized for 3 h at 950 °C. Activation of carbonized aerogel was performed at two different times: for 1 h termed as low activated HCAM, and 3 h termed as high activated HCAM. A low-activated HCAM exhibits a sodium ion selectivity of SNa/Ca ≫10 over calcium ions at applied potential 0.6–1.2 V for 5 mM NaCl and 2.5 mM CaCl2 solutions. On the other hand, highly activated HCAM displayed a selectivity of 6.6±0.8 at 0.6 V calcium ions which decreases to 2.2±0.03 with raise in the potential to 1.2 V, against the sodium ions.
7.2 Temperature
The pore structure and morphological properties of the electrode material varied at different temperatures of activation. For example, Zornitta et al. prepared para-toluene sulfonic acid (Pts) doped PANI/Pts polymer followed by carbonization performed at different temperatures (200–850 °C) under inert atmosphere.140 The carbonized samples were activated using KOH at 850 °C for 1.5 h. The samples carbonized at 500–600 °C and activated at 850 °C exhibit a high surface area of 3649 m2 g−1, a specific capacitance of 213 F g−1, and SAC of 22.2 mg g−1, owing to the oxygen-rich carbons and turbo-static structure. On the contrary, activation of carbonized samples at 900 °C led to a stable pore structure, however, due to pore shrinkage and pore disintegration phenomenon the pore volume and porosity of the structure were compromised. Generally, the electrodes after CDI are regenerated via polarity reversal or short circuits. The desorption rate for the absorption-desorption cycle can be evaluated from desorption mode or electrode regeneration mode. Huang et al. have investigated the effect of temperature (15–45 °C) of salt solution and desorption mode in CDI application.141 According to the experiments, the higher temperature lead to low SAC value due to reduced electrode-ion affinity and an increase in ion repulsion from the electrode.
7.3 Doping agents
The molecular size and mass fraction of the dopants could lend a massive impact on the ionic and electronic conductivity of the electrode materials. In this line, Reale et al. investigated the effect of two conductive additives and insulating active species namely i) C45 with a surface area of 45 m2 g−1 and particle radii of 960 nm, and ii) Ketjen black EC-600JX (KB) with a surface area of 1270 m2 g−1 and radii of 34 nm, with alumina on their electrosorption performance.142 The KB displayed superior electrical conductivity at lower mass fraction than conventional carbon black generally applied in CDI. This is due to its high specific surface area where the small particle radii lead to fast ion diffusion.
7.4 Experimental modes
The electrode orientation can affect the electrosorption performance of monovalent ions removal in CDI application. The electrode can be placed cathode upstream and anode downstream or vice versa. Algurainy et al. have investigated the effect of electrode orientation on the removal of sodium and chloride ions and oxygen reduction reactions (ORRs).143 According to their investigation, the upstream cathode orientation leads to symmetric electrosorption of both the ion where upstream anode orientation resulted in the 2.5 times higher removal of sodium ion. This reduction in electrosorption of sodium ions was attributed to the H+ generated due to the oxidation of carbon at anode and enhanced ORRs at the cathode downstream. However, the removal of chloride ions during both orientation modes was found equal.
7.5 Electrode structure degradation
Typically, porous carbon electrodes utilized in CDI suffer from the limitation of structural degradation due to the parasitic redox reaction on application of voltage. Zornitta et al. prepared PANI coated porous carbon electrodes and performed anion uptake in multi-channel mCDI and ion removal in CDI.144 Application of low potential +0.35 V (vs Ag/AgCl) attains chloride ion removal capacity of 65 mg g−1 and charge efficiency of 100 % for CDI. However, an increment in the applied potential to 0.60 and 0.80 V (vs Ag/AgCl) was found to decrease the columbic efficiency to 90 % and 76 % (after 10 cycles), respectively. Lowering down of columbic efficiency with an increase in applied voltage was attributed to the oxidation of PANI. For a solution containing Cl-, H2PO4−, and SO42− ions, the PANI-coated carbon electrode displayed selective removal of Cl- over H2PO4−, and SO42− ions, over the pristine electrode.
7.6 Feed water constituents
Shim et al. evaluated the effect of two natural organic matters i.e. humic acid (HA) and tannic acid (TA) on the electrosorption performance of removal of sodium chloride and calcium chloride in mCDI application.145 According to the results, HA forms a charge-neutralized complex with CaCl2 which marginally affect the removal efficiency of calcium ion. On the other hand, HA disturbs the absorption of sodium ion process and significantly decreases salt removal by 68 %. The presence of TA lowers the removal of both NaCl and CaCl2 by 37 and 60 %, respectively, due to lower hydrodynamic diameter which causes strong adhesion to pores present in the electrode material. Wang et al., performed electrosorption performance using two solutions: one with powdered AC (PAC) mixed with HA in salt solution, and second without PAC.146 The salt solution with PAC exhibited improved SAC and a decrease in HA deposition by ≈40 %. In addition, an improved electrical conductivity by EDL and decrease in internal resistance of CDI was observed. In another report, Wang et al. studied the effect of model foulants i.e. organic matter (bovine serum albumin, BSA) and inorganic matter (CaCl2) on the desalination performance of mCDI in long-term operation.147 Here, the concentration of calcium plays a critical role in controlling the fouling effect of BSA. For 0.5–3 mM CaCl2 solution, the deposition of BSA was reduced from 1.72 to 0.71 μg cm−3. On the contrary, increasing the concentration to 5 mM the BSA deposition reached 1.02 μg cm−3. This could be attributed to the variation in the size distribution. With an increase in the calcium ion concentration, the calcium content on the membrane surface increased. As a result, the effect of BSA reduce while the scaling effect became the main factor for efficiency lowering.
8 Green and sustainable electrode/membrane materials
The utilization of bio-based materials as cost-effective and energy-efficient electrode materials for the purification of water has garnered substantial research interest.148 Several methods including the impregnation of electrospinning mats, vacuum filtering, and direct deposition of a solution have been developed for fabricating bio-based membranes. For example, Huang et al. created transparent lipid nanoporous membranes (TFN NFMs) by hydrolyzing microcrystalline cellulose nanocrystals with sulfuric acid.149 The addition of nanocrystals in the membrane has increased the water flow from 78.9 L m−2 h−1 to 106.9 L m−2 h−1 while improving chlorine resistance. Kayani et al. prepared a chitosan membrane made of polyethylene glycol (PEF-600) that was cross-linked with 3-aminopropyltriethoxysilane.150 The reverse osmosis filtration findings showed an increase in the water flow of 80 % and a salt rejection value of 40.4 %.
The development of sustainable carbon fiber from bio-based sources for use as an electrode material for water CDI/mCDI is a good choice.151, 152 At present, polyacrylonitrile is utilized to make more than 90 % of carbon fibers.152 Nevertheless, the high cost of raw ingredients restricts its use to high-value items. The cheaper bio-derived precursors are a viable way to address carbon nanofiber cost and sustainability. Baker et al. calculated the cost of lignin-based carbon nanofibers at roughly 6.3 USD per kg with a 35 % carbon output.153 The costs might be further reduced if carbon yield is enhanced by improving production conditions. Notably, worldwide research activities, such as the Eu(WoCaFi),152 Libre 2020,151 Greenlight,154 USA,155 and Japan156 programs, are undertaken to commercialize bio-derived carbon fibers.
9 Summary and future prospects
Capacitive deionization has been identified as one of the promising techniques for water desalination. A significant amount of effort is increasingly being invested in developing CDI/mCDI cell architectures for better desalination systems and high-performance electrode materials. In this review, we have briefly explained the CDI-mCDI cell architectures and the newly investigated electrode materials for the CDI/mCDI applications. A conventional CDI system consists of two electrodes made of the same material and the feed water flows between them. On application of voltage, an electric field is developed between the electrodes by which ions move towards the opposite charge electrodes. As a result, the ions are absorbed in the pores of the electrodes and later removed during the regeneration process of the electrodes and in this way the feed water is desalinated. However, the process suffers from the co-ion expulsion effect which degrades the electrode life and depletes the water desalination performance of the material. The co-ion expulsion effect can be easily minimized by placing IEMs in front of the electrodes which increases the diffusion of salt ions and ultimately improve the electrosorption performance and the longevity of the electrodes. Among the various CDI/mCDI cell architectures, the FB-CDI/mCDI, FT-CDI, i-CDI, and flow-electrode CDI cell systems involve electrostatic and capacitive mechanism, i.e. non-faradaic processes, while the hybrid CDI and desalination battery involves reversible redox reaction mechanism, i.e. faradaic process, for salt ion removal for the desalination process.
Owing to the chemical robustness, high specific surface area, and accessible pore size distribution, porous carbon-based materials are ideal for the water desalination process. An ion diffusion pathway provided by the structure of electrode material for salt ions is always critical and straight away influences the desalination performance and salt ion removal capacity of the material utilized for CDI/mCDI desalination application. However, the synergistic effect of micropores and mesopores controlling the diffusion capacity of materials has been poorly considered and even less evaluated through green and sustainable approaches. There are some reports where the conductivity and SAC of the material are improved by doping with hetero atoms. In some cases, biomass-derived green carbon materials are used to make the approach more sustainable. Graphene and graphene composites served as another option for potential electrode materials to improve the electrosorption performance. In addition, polymer and polymer composites doped with metal or metal oxide displayed improved SAC values, owing to the pseudocapacitive behavior. On the other hand, MXenes displayed a much better performance exhibiting SAC values up to 130 mg g−1. This is attributed to the highly conjugated system, high conductivity, and pseudocapacitance behavior acquired by the material via the presence of metal or metal oxide in the structure. Unfortunately, the high cost and complicated processing technology are some of the hurdles which limit the application of MXenes for water desalination on industrial scale. The electrosorption performance of the CDI/mCDI also depends on several factors such as temperature, dopants, operational condition, feed water composition, and materials used in the electrodes. In addition to this, several other factors must be addressed while evaluating the IEMs desalination performance in CDI/mCDI. For example, chemical stability, fabrication method, cost, large-scale production, environmental sustainability, recyclability, stability over multiple desalination cycles, and R-factor describing the relative improvement in desalination performance of mCDI compared to CDI. By default, owing to low energy input the mCDI is more environmentally sustainable desalination technology compared to energy-intensive processes such as distillation. Apart from this, it should be ensured that the materials, solvents, cross-linkers, and electrodes utilized for mCDI are sustainable. In this line, the use of bio-based green solvents as an alternative to toxic organic solvents for the fabrication of polymer IEMs could represent a sustainable mCDI technology. In terms of water desalination performance, high SAC and chemical efficiency with permselectivity value closer to 1 represent the high electrosorption performance of the IEMs. Among IEMs, AEM plays a major role in minimizing the co-ion expulsion effect and protects the degradation of the electrode by anodic oxidation reactions. In this context, more efforts should be invested to develop and engineer AEMs to ensure the longevity of the electrodes and the high salt ion removal capacity in mCDI.
Notably, regardless of the types of electrode materials or IEMs utilized in CDI/mCDI, there is a great inconsistency between the experimental parameters describing the performance. The data normalization typically involves expressing the CDI/mCDI performance data using a common set of units and normalization factors, such as electrode area or charge passed, depending on the specific research goals. This allows for easier comparison of different ion exchange materials and helps to identify the best-performing materials for future water desalination studies. By establishing a standard approach for reporting CDI/mCDI data, researchers can achieve greater consistency and accuracy in their results. For example, salt ions removal and charge efficiency of the ion exchange materials in mCDI should be explicitly compared with CDI findings. This will help the readers to better understand the effect on the overall desalination performance by introducing of IEMs. The concentration of feed salt used in desalination is a variable that differs widely among research. Yet, the amount of the saline solution might have an impact on estimated performance parameters like SAC. To make it easier to compare different reports, some standard feed concentrations must be chosen for CDI/mCDI experiments. In addition to this, a benchmark applied voltage should be set for all CDI/mCDI experiments to establish a fair performance comparison. As we have seen that the SAC value significantly improves with an increase in the applied voltage and feed saltwater concentration,157 to study long-term performance either multiple cycles or hours of operation should be performed for every new ion exchange material developed for CDI/mCDI. The prolonged proficiency of IEMs is an aspect that is frequently disregarded whilst assessing the efficacy of CDI/mCDI. In this aspect, the cycle stability of a CDI/mCDI system should be prioritized alongside SAC, and separation efficiency such as applied potential, current, and energy input. It is critical to assess performance throughout several desalination cycles; single-cycle findings are not indicative of the entire system capacity. Thus, rigorous testing is required to assure cycle stability while developing any new IEMs for water desalination.
Abbreviations
-
CDI
-
Capacitive Deionization;
-
mCDI
-
Membrane Capacitive Deionization;
-
RO
-
Reverse Osmosis;
-
ED
-
Electrodialysis;
-
IEMs
-
Ion Exchange Membranes;
-
CEM
-
Cation-Exchange Membrane;
-
AEM
-
Anion Exchange Membrane;
-
i-CDI
-
An Inverted-CDI;
-
EPZC
-
Potential Of Zero Charge;
-
CNTs
-
Carbon Nanotubes;
-
SAC
-
Salt Absorption Capacity;
-
ASAR
-
Average Salt Absorption Rate;
-
CE
-
Charge Efficiency;
-
DC
-
Desorption Capacity;
-
ERE
-
Electrode Regeneration Efficiency;
-
PPC
-
PVDF-Derived Porous Carbons;
-
AC
-
Activated Carbon;
-
DES
-
Deep Eutectic Solvent;
-
COF
-
Covalent Organic Frameworks;
-
PCFs
-
Porous Carbon Fiber;
-
PMMA-b-PAN
-
Polymethyl Methacrylate-Block-Polyacrylonitrile;
-
PCNFs
-
Porous Carbon Nanofibers;
-
PCNs
-
Porous Activated Carbon Nanoflakes;
-
NiHCF
-
Nickel Hexacyanoferrate;
-
GNS
-
Graphene Oxide/Cnts Sponge;
-
PCP
-
Porous Carbon Polyhedral;
-
HBC
-
Hollow Bowl-Like Carbon;
-
PB
-
Prussian Blue;
-
Ppy
-
Polypyrrole;
-
PANI
-
Polyaniline;
-
PTP
-
Polythiophene;
-
NPPC
-
nitrogen-doped porous carbon;
-
CCS/GCNS
-
Chitosan-Based Carbon Electrode;
-
NPPC
-
Nitrogen-Doped Porous Carbon;
-
WCA
-
Water Contact Angle;
-
IEC
-
Ion-Exchange Capacity;
-
PVDF
-
Polyvinylendene Polymer;
-
PPC
-
PVDF-Derived Porous Carbon;
-
QPBI
-
Quarternized Polybenzimidazole;
-
PCS
-
Porous Carbon Spheres;
-
MT
-
Montmorillonite;
-
HT
-
Hydrotalcite;
-
HCAM
-
Hierarchical Carbon Aerogel Monolith;
-
Pts
-
Para-Toluene Sulfonic Acid;
-
KB
-
Ketjen Black EC-600JX;
-
ORRs
-
Oxygen Reduction Reactions;
-
HA
-
Humic Acid;
-
PAC
-
Powdered Activated Carbon;
-
BSA
-
Bouvine Serum Albumin;
-
ALD
-
Active Layer Deposition.
Acknowledgments
DS acknowledges Khalifa University Abu Dhabi for its generous support. This work is supported by Khalifa University Competitive Internal Research Award (CIRA-2022). NMA thanks Khalifa University for Doctoral Research Teaching Scholarship (DRTS).
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Biographical Information
Sushil Kumar received his Ph.D. degree (2014) in chemistry from the University of Delhi. He pursued his postdoctoral research at National Chemical Laboratory, India, and later moved to King Abdullah University of Science and Technology in 2019 as a postdoc. Currently, he is a Research Scientist in Dr. Shetty's research group at Khalifa University. Lately, his main research interest is the design and synthesis of COFs-derived electrodes for capacitive deionization applications.
Biographical Information
Dr. Emad Alhseinat is an Associate Professor at Khalifa University's Department of Chemical Engineering. His research focuses on addressing the issue of freshwater scarcity in arid regions by enhancing the efficiency of water desalination and treatment processes and developing creative and sustainable solutions. He has expertise in capacitive deionization electrode materials, as well as process modeling and optimization.
Biographical Information
Najat Maher Aldaqqa is a Ph.D. candidate under the supervision of Dr. Dinesh Shetty in the Department of Chemistry at Khalifa University. Her main research interest is the design and synthesis of redox-active COFs-derived electrode materials for water desalination applications.
Biographical Information
Dr. Dinesh Shetty holds a Ph.D. degree (2011) in chemistry from Seoul National University, Korea. He pursued postdoctoral research at Emory University, USA, and Institute for Basic Science, POSTECH, Korea, before moving to New York University Abu Dhabi to work as a research scientist. In 2019, he started an independent career at Khalifa University, Abu Dhabi. His research interest is focused on developing multifunctional materials for various applications, including energy and water purification.








