Experimental Detection of Trinitramide, N(NO2)3†
Corresponding Author
Dr. Martin Rahm
Physical Chemistry, School of Chemical Science and Engineering, Royal Institute of Technology (KTH), 100 44 Stockholm (Sweden), Fax: (+46) 8-790-8207
Competence Centre for Energetic Materials (KCEM), Gammelbackavägen 6, 69151 Karlskoga (Sweden)
Physical Chemistry, School of Chemical Science and Engineering, Royal Institute of Technology (KTH), 100 44 Stockholm (Sweden), Fax: (+46) 8-790-8207Search for more papers by this authorDr. Sergey V. Dvinskikh
Physical Chemistry, School of Chemical Science and Engineering, Royal Institute of Technology (KTH), 100 44 Stockholm (Sweden), Fax: (+46) 8-790-8207
Search for more papers by this authorProf. István Furó
Physical Chemistry, School of Chemical Science and Engineering, Royal Institute of Technology (KTH), 100 44 Stockholm (Sweden), Fax: (+46) 8-790-8207
Search for more papers by this authorCorresponding Author
Prof. Tore Brinck
Physical Chemistry, School of Chemical Science and Engineering, Royal Institute of Technology (KTH), 100 44 Stockholm (Sweden), Fax: (+46) 8-790-8207
Physical Chemistry, School of Chemical Science and Engineering, Royal Institute of Technology (KTH), 100 44 Stockholm (Sweden), Fax: (+46) 8-790-8207Search for more papers by this authorCorresponding Author
Dr. Martin Rahm
Physical Chemistry, School of Chemical Science and Engineering, Royal Institute of Technology (KTH), 100 44 Stockholm (Sweden), Fax: (+46) 8-790-8207
Competence Centre for Energetic Materials (KCEM), Gammelbackavägen 6, 69151 Karlskoga (Sweden)
Physical Chemistry, School of Chemical Science and Engineering, Royal Institute of Technology (KTH), 100 44 Stockholm (Sweden), Fax: (+46) 8-790-8207Search for more papers by this authorDr. Sergey V. Dvinskikh
Physical Chemistry, School of Chemical Science and Engineering, Royal Institute of Technology (KTH), 100 44 Stockholm (Sweden), Fax: (+46) 8-790-8207
Search for more papers by this authorProf. István Furó
Physical Chemistry, School of Chemical Science and Engineering, Royal Institute of Technology (KTH), 100 44 Stockholm (Sweden), Fax: (+46) 8-790-8207
Search for more papers by this authorCorresponding Author
Prof. Tore Brinck
Physical Chemistry, School of Chemical Science and Engineering, Royal Institute of Technology (KTH), 100 44 Stockholm (Sweden), Fax: (+46) 8-790-8207
Physical Chemistry, School of Chemical Science and Engineering, Royal Institute of Technology (KTH), 100 44 Stockholm (Sweden), Fax: (+46) 8-790-8207Search for more papers by this authorWe gratefully acknowledge support given by the Swedish Research Council (VR), the Swedish Defence Research Agency (FOI), and Eurenco Bofors. Exselent and the Knut and Alice Wallenberg foundation are thanked for the IR equipment. Michael Holmboe, Madeleine Warner, and Henrik Skifs are thanked for their kind assistance.
Graphical Abstract
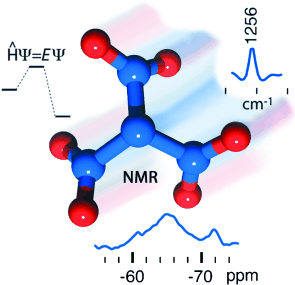
Treibstoff in Propellerform: Das bislang größte Stickstoffoxid, Trinitramid (TNA), wurde IR- und NMR-spektroskopisch nachgewiesen, nachdem in quantenchemischen Studien seine kinetische Beständigkeit und einige physikalische Eigenschaften vorhergesagt worden waren. Die Verbindung ist außerordentlich energiereich und möglicherweise als kryogener Treibstoff und zur Erforschung von Materialien mit hoher Energiedichte geeignet.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201007047_sm_miscellaneous_information.pdf1.7 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. Giles, Nature 2004, 427, 580–581.
- 2R. P. Singh, R. D. Verma, D. T. Meshri, J. M. Shreeve, Angew. Chem. 2006, 118, 3664–3682; Angew. Chem. Int. Ed. 2006, 45, 3584–3601.
- 3M. B. Talawar, R. Sivabalan, T. Mukundan, H. Muthurajan, A. K. Sikder, B. R. Gandhe, A. S. Rao, J. Hazard. Mater. 2009, 161, 589–607.
- 4K. O. Christe, W. W. Wilson, J. A. Sheehy, J. A. Boatz, Angew. Chem. 1999, 111, 2112–2118;
10.1002/(SICI)1521-3757(19990712)111:13/14<2112::AID-ANGE2112>3.0.CO;2-I Google ScholarAngew. Chem. Int. Ed. 1999, 38, 2004–2009.10.1002/(SICI)1521-3773(19990712)38:13/14<2004::AID-ANIE2004>3.0.CO;2-7 CAS PubMed Web of Science® Google Scholar
- 5R. Haiges, S. Schneider, T. Schroer, K. O. Christe, Angew. Chem. 2004, 116, 5027–5032;
10.1002/ange.200454242 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 4919–4924.
- 6T. M. Klapötke, J. Stierstorfer, J. Am. Chem. Soc. 2009, 131, 1122–1134.
- 7A. Vij, J. G. Pavlovich, W. W. Wilson, V. Vij, K. O. Christe, Angew. Chem. 2002, 114, 3177–3180;
10.1002/1521-3757(20020816)114:16<3177::AID-ANGE3177>3.0.CO;2-I Google ScholarAngew. Chem. Int. Ed. 2002, 41, 3051–3054.10.1002/1521-3773(20020816)41:16<3051::AID-ANIE3051>3.0.CO;2-T CAS PubMed Web of Science® Google Scholar
- 8H. Östmark, S. Wallin, T. Brinck, P. Carlqvist, R. Claridge, E. Hedlund, L. Yudina, Chem. Phys. Lett. 2003, 379, 539–546.
- 9O. A. Luk′yanov, Y. V. Konnova, T. A. Klimova, V. A. Tartakovsky, Izv. Akad. Nauk Ser. Khim. 1994, 1264–1266.
- 10J. C. Bottaro, P. E. Penwell, R. J. Schmitt, Synth. Commun. 1991, 21, 945–949.
- 11R. Gilardi, J. Flippen-Anderson, C. George, R. J. Butcher, J. Am. Chem. Soc. 1997, 119, 9411–9416.
- 12E. A. Miroshnichenko, L. I. Korchatova, B. L. Korsunskii, B. S. Fedorov, Y. D. Orlov, L. T. Eremenko, Y. A. Lebedev, F. I. Dubovitskii, Dokl. Akad. Nauk SSSR 1987, 295, 419–423.
- 13J. A. Montgomery, Jr., H. H. Michels, J. Phys. Chem. 1993, 97, 6774–6775.
- 14Z. Chen, T. P. Hamilton, J. Phys. Chem. A 1999, 103, 11026–11033.
- 15A. I. Kazakov, Y. I. Rubtsov, G. B. Manelis, L. P. Andrienko, Russ. Chem. Bull. 1997, 46, 2015–2020.
- 16M. Rahm, T. Brinck, J. Phys. Chem. A 2010, 114, 2845–2854.
- 17J. S. Murray, T. Brinck, P. Politzer, Chem. Phys. 1996, 204, 289–299.
- 18P. Politzer, J. S. Murray, J. Phys. Chem. A 1998, 102, 1018–1020.
- 19M. Rahm, T. Brinck, Chem. Eur. J. 2010, 16, 6590–6600.
- 20J. P. Ritchie, E. A. Zhurova, A. Martin, A. A. Pinkerton, J. Phys. Chem. B 2003, 107, 14576–14589.
- 21J. Kowalewski, Mäler, L., Nuclear Spin Relaxation in Liquids: Theory, Experiments, and Applications, Taylor and Francis, New York, 2006.
- 22M. Rahm, T. Brinck, Chem. Commun. 2009, 20, 2896–2898.
- 23X. Zhang, K. Seppelt, Z. Anorg. Allg. Chem. 1998, 624, 667–670.
- 24A. Haussler, T. M. Klapötke, H. Piotrowski, Z. Naturforsch. B 2002, 57, 151–156.
- 25V. M. Mastikhin, S. V. Filimonova, J. Chem. Soc. Faraday Trans. 1992, 88, 1473–1476.
- 26V. A. Shlyapochnikov, M. A. Tafipolsky, I. V. Tokmakov, E. S. Baskir, O. V. Anikin, Y. A. Strelenko, O. A. Luk′yanov, V. A. Tartakovsky, J. Mol. Struct. 2001, 559, 147–166.
- 27A. A. Lobanova, S. G. Il′yasov, N. I. Popov, R. R. Sataev, Russ. J. Org. Chem. 2002, 38, 1–6.
- 28J. A. Montgomery, Jr., M. J. Frisch, J. W. Ochterski, G. A. Petersson, J. Chem. Phys. 1999, 110, 2822–2827.
- 29Gaussian 03 (Revision C.02), M. J. Frisch, et al., Gaussian, Inc. Wallingford, CT, 2004. See S. I. for full list of authors.
- 30S. Grimme, J. Chem. Phys. 2006, 124, 034108.
- 31F. Neese, T. Schwabe, S. Grimme, J. Chem. Phys. 2007, 126, 124115.
- 32T. Brinck, Hardsurf program. (HS95v09), 2009.
- 33B. M. Rice, S. V. Pai, J. Hare, Combust. Flame 1999, 118, 445–458.
- 34B. J. McBride, S. Gordon, Computer Program for Calculation of Complex Chemical Equilibrium Compositions and Applications. II. Users Manual and Program Description, NASA, 1996.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.