Resurrecting the Cornforth Model for Carbonyl Addition: Studies on the Origin of 1,2-Asymmetric Induction in Enolate Additions to Heteroatom-Substituted Aldehydes†
David A. Evans Prof.
Department of Chemistry and Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617-495-1460
Search for more papers by this authorSarah J. Siska
Department of Chemistry and Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617-495-1460
Search for more papers by this authorVictor J. Cee
Department of Chemistry and Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617-495-1460
Search for more papers by this authorDavid A. Evans Prof.
Department of Chemistry and Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617-495-1460
Search for more papers by this authorSarah J. Siska
Department of Chemistry and Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617-495-1460
Search for more papers by this authorVictor J. Cee
Department of Chemistry and Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617-495-1460
Search for more papers by this authorFinancial support was provided by the National Institutes of Health (GM33327-16) and by Merck Research Laboratories.
Graphical Abstract
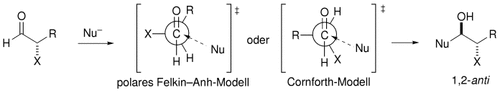
Weitere experimentelle Bestätigung für das Cornforth-Modell wurde im Rahmen einer Untersuchung der Aldolreaktion von Methyl-substituierten E- und Z-Enolaten mit α-Sauerstoff-substituierten Aldehyden erhalten. Die beobachtete Abhängigkeit der Diastereoseitenselektivität von der Konfiguration des Enolats ist eher mit dem Cornforth-Modell in Einklang als mit dem polaren Felkin-Anh-Modell (siehe Schema; X=OR, NR2, Cl).
References
- 1A. Mengel, O. Reiser, Chem. Rev. 1999, 99, 1191–1223, and references therein.
- 2D. J. Cram, F. A. B. Elhafez, J. Am. Chem. Soc. 1952, 74, 5828–5835.
- 3
- 3aJ. W. Cornforth, R. H. Cornforth, K. K. Mathew, J. Chem. Soc. 1959, 112–127;
- 3bD. J. Cram, D. R. Wilson, J. Am. Chem. Soc. 1963, 85, 1245–1249;
- 3cG. J. Karabatsos, J. Am. Chem. Soc. 1967, 89, 1367–1371;
- 3dM. Chérest, H. Felkin, N. Prudent, Tetrahedron Lett. 1968, 2199–2204. For additional discussion of some of these models, see:
- 3eE. L. Eliel in Asymmetric Synthesis, Vol. 2 (Ed.: ), Academic Press, New York, 1983, pp. 125–155. For a discussion of electrostatic versus frontier molecular orbital (FMO) effects in carbonyl additions, see:
10.1016/B978-0-12-507702-6.50010-4 Google Scholar
- 3fB. W. Gung, Tetrahedron 1996, 52, 5263–5301.
- 4
- 4aN. T. Anh, O. Eisenstein, Nouv. J. Chim. 1977, 1, 61–70;
- 4bN. T. Anh, Top. Curr. Chem. 1980, 88, 145–162.
10.1007/BFb0048506 Google Scholar
- 5For theoretical studies which support the polar Felkin–Anh model, see:
- 5aY.-D. Wu, K. N. Houk, J. Am. Chem. Soc. 1987, 109, 908–910;
- 5bS. S. Wong, M. N. Paddon-Row, J. Chem. Soc. Chem. Commun. 1990, 456–458;
- 5cY.-D. Wu, J. A. Tucker, K. N. Houk, J. Am. Chem. Soc. 1991, 113, 5018–5027;
- 5dS. S. Wong, M. N. Paddon-Row, J. Chem. Soc. Chem. Commun. 1991, 327–330;
- 5eS. S. Wong, M. N. Paddon-Row, Aust. J. Chem. 1991, 44, 765–770;
- 5fG. Frenking, K. F. Köhler, M. T. Reetz, Tetrahedron 1991, 47, 9005–9018;
- 5gG. Frenking, K. F. Köhler, M. T. Reetz, Tetrahedron 1993, 49, 3983–3994;
- 5hN. T. Anh, F. Maurel, J.-M. Lefour, New J. Chem. 1995, 19, 353–364.
- 6The Cornforth model has been modified from its original form to incorporate torsional effects and the Bürgi–Dunitz trajectory. For a discussion of torsional effects in transition states, see: M. N. Paddon-Row, N. G. Rondan, K. N. Houk, J. Am. Chem. Soc. 1982, 104, 7162–7166.
- 7Addition of cyanide anion to fluoroacetaldehyde in solution:
- 7aA. S. Cieplak, K. B. Wiberg, J. Am. Chem. Soc. 1992, 114, 9226–9227. Addition of cyanide anion to chlorofluoroacetaldehyde in the gas phase:
- 7bG. Frenking, K. F. Köhler, M. T. Reetz, Tetrahedron 1994, 50, 11 197–11 204. Allylboration of α-methoxypropanal in the gas phase:
- 7cB. W. Gung, X. Xue, Tetrahedron: Asymmetry 2001, 12, 2955–2959.
- 8
- 8aR. W. Hoffmann, Chem. Scr. 1985, 25, 53–60 (Sp. Iss.);
- 8bW. R. Roush, M. A. Adam, A. E. Walts, D. J. Harris, J. Am. Chem. Soc. 1986, 108, 3422–3434;
- 8cR. W. Hoffmann, R. Metternich, J. W. Lanz, Liebigs Ann. Chem. 1987, 881–887;
- 8dH. Brinkmann, R. W. Hoffmann, Chem. Ber. 1990, 123, 2395–2401.
- 9
- 9aD. A. Evans, J. V. Nelson, E. Vogel, T. R. Taber, J. Am. Chem. Soc. 1981, 103, 3099–3111. For an extensive review of boron enolate based aldol additions see:
- 9bC. J. Cowden, I. Paterson, Org. React. 1997, 51, 1–200.
- 10This effect is well known for α-methyl chiral aldehydes. See:
- 10aD. A. Evans, J. V. Nelson, T. R. Taber, Top. Stereochem. 1982, 13, 1–115;
- 10bW. R. Roush, J. Org. Chem. 1991, 56, 4151–4157.
- 11Boron enolates:
- 11aH. C. Brown, K. Ganesan, R. K. Dhar, J. Org. Chem. 1993, 58, 147–153; Z lithium enolate:
- 11bC. H. Heathcock, C. T. Buse, W. A. Kleschick, M. C. Pirrung, J. E. Sohn, J. Lampe, J. Org. Chem. 1980, 45, 1066–1081; E lithium enolate:
- 11cL. Xie, K. M. Isenberger, G. Held, L. M. Dahl, J. Org. Chem. 1997, 62, 7516–7519.
- 12In the case of the boron aldol reactions, it is possible that the difference in aldehyde diastereofacial selectivity between E and Z enolates is simply due to differences in the nature of the boron ligands that are necessary for the control of enolate configuration. However, both 9-BBN and (c-Hex)2B enolates of cyclohexanone give similar results, indicating that enolate configuration is primarily responsible for the selectivity difference.
- 13Lithium enolates:
- 13aC. H. Heathcock, S. D. Young, J. P. Hagen, M. C. Pirrung, C. T. White, D. VanDerveer, J. Org. Chem. 1980, 45, 3846–3856. Boron enolates:
- 13bC. Gennari, A. Bernardi, S. Cardani, C. Scolastico, Tetrahedron 1984, 40, 4059–4065;
- 13cD. R. Williams, J. L. Moore, M. Yamada, J. Org. Chem. 1986, 51, 3916–3918. Titanium enolates:
- 13dR. Annunziata, M. Cinquini, F. Cozzi, P. G. Cozzi, E. Consolandi, Tetrahedron 1991, 47, 7897–7910;
- 13eC. Esteve, M. Ferreró, P. Romea, F. Urpí, J. Vilarrasa, Tetrahedron Lett. 1999, 40, 5079–5082. Samarium enolates:
- 13fL. Lu, H.-Y. Chang, J.-M. Fang, J. Org. Chem. 1999, 64, 843–853.
- 14In contrast, crotylation of α-chiral oxygen-substituted aldehydes with crotyl bromide and CrCl2 is reported to proceed with good to excellent selectivity for the 2,3-anti, 3,4-anti diastereomer. See:
- 14aS. F. Martin, W. Li, J. Org. Chem. 1989, 54, 6129–6133;
- 14bJ. Mulzer, L. Kattner, A. R. Strecker, C. Schröder, J. Buschmann, C. Lehmann, P. Luger, J. Am. Chem. Soc. 1991, 113, 4218–4229.
- 15The matched/mismatched relationship between Z and E chiral γ-chloroallylborane reagents and α-chiral oxygen-substituted aldehydes is also consistent with Cornforth transition states (Figure 1, III and IV). See: S. Hu, S. Jayaraman, A. C. Oehlschlager, J. Org. Chem. 1998, 63, 8843–8849.
- 16Abbreviations: PFA=polar Felkin–Anh; DIPEA=diisopropylethylamine; 9-BBNOTf=9-borabicyclo[3.3.1]nonyl trifluoromethanesulfonate; TEA=triethylamine; LiHMDS=lithium hexamethyldisilazide; TMS=trimethylsilyl; THF=tetrahydrofuran; Bn=benzyl; TBS=tert-butyldimethylsilyl; pyr=pyridine; LDA=lithium diisopropylamide; TBDPS=tert-butyldiphenylsilyl.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.