Sequence-Selective Molecular Recognition between β Sheets†
James S. Nowick Prof.
Department of Chemistry, University of California, Irvine, Irvine, CA 92697-2025, USA, Fax: (+1) 949-824-9920
Search for more papers by this authorDe Michael Chung
Department of Chemistry, University of California, Irvine, Irvine, CA 92697-2025, USA, Fax: (+1) 949-824-9920
Search for more papers by this authorJames S. Nowick Prof.
Department of Chemistry, University of California, Irvine, Irvine, CA 92697-2025, USA, Fax: (+1) 949-824-9920
Search for more papers by this authorDe Michael Chung
Department of Chemistry, University of California, Irvine, Irvine, CA 92697-2025, USA, Fax: (+1) 949-824-9920
Search for more papers by this authorWe thank the NIH for grant support (GM-49076). D.M.C. thanks the Bill and Melinda Gates Foundation and the UCI Institute for Brain Aging and Dementia for a fellowship and training grant support (NIA-5T32AG000096).
Graphical Abstract
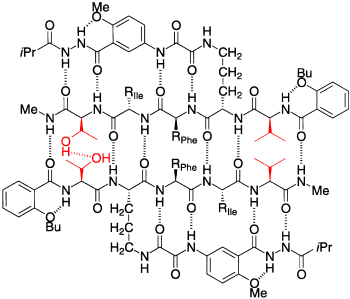
Sequenzselektive molekulare Erkennung zwischen Peptiden mit β-Faltblatt-Sekundärstruktur: Lediglich durch gezielte Positionierung zweier Threonin(Thr)- oder Valin(Val)-Reste lassen sich Peptide erhalten, die bevorzugt entweder Homodimere oder Heterodimere bilden. Gezeigt ist die Struktur eines Thr-Val⋅Val-Thr-Heterodimers.
References
- 1S. Maitra, J. S. Nowick in The Amide Linkage: Structural Significance in Chemistry, Biochemistry, and Materials Science (Ed ), Wiley, New York, 2000, pp. 495–518.
- 2C. Petosa, R. J. Collier, K. R. Klimpel, S. H. Leppla, R. C. Liddington, Nature 1997, 385, 833–838.
- 3For related observations in proteins associated with genetic diseases, see:
- 3aP. Hammarström, F. Schneider, J. W. Kelly, Science 2001, 293, 2459–2462;
- 3bE. G. Huizinga, S. Tsuji, R. A. P. Romijn, M. E. Schiphorst, P. G. de Groot, J. J. Sixma, P. Gros, Science 2002, 297, 1176–1179.
- 4J. S. Nowick, K. S. Lam, T. V. Khasanova, W. E. Kemnitzer, S. Maitra, H. T. Mee, R. Liu, J. Am. Chem. Soc. 2002, 124, 4972–4973.
- 5
- 5aS. Lifson, C. Sander, J. Mol. Biol. 1980, 139, 627–639;
- 5bM. A. Wouters, P. M. G. Curmi, Proteins Struct. Funct. Genet. 1995, 22, 119–131;
- 5cE. G. Hutchinson, R. B. Sessions, J. M. Thornton, D. N. Woolfson, Protein Sci. 1998, 7, 2287–2300;
- 5dY. Mandel-Gutfreund, S. M. Zaremba, L. M. Gregoret, J. Mol. Biol. 2001, 305, 1145–1159.
- 6Studies were performed at 253 K. Although the resonance signals are resolved in the 500 MHz 1H NMR spectrum at 298 K, they are sharper at lower temperatures. Measurement of the relative concentrations of the homo- and heterodimers at varying ratios of peptides verifies that KHet is invariant and supports its use to gauge the preferences of amino acid pairings.
- 7Further addition of [D6]DMSO beyond 10 % results in additional increases in selectivity, however shimming of the NMR instrument becomes difficult at higher [D6]DMSO concentrations. In pure [D6]DMSO, dimers do not form.
- 8B. Kirchner, M. Reiher, J. Am. Chem. Soc. 2002, 124, 6206–6215, and references therein.
- 9
- 9aT.-L. Hwang, A. J. Shaka, J. Am. Chem. Soc. 1992, 114, 3157–3159;
- 9bT.-L. Hwang, A. J. Shaka, J. Magn. Reson. Ser. B 1993, 102, 155–165.
- 10NOEs between the Thr α-protons of the heterodimer could not be observed because the resonance signals of these protons are nearly coincident.
- 11
- 11aC. K. Smith, L. Regan, Science 1995, 270, 980–982;
- 11bC. A. Blasie, J. M. Berg, Biochemistry 1997, 36, 6218–6222.
- 12A. Horovitz, Folding Des. 1996, 1, R 121-R126.
- 13M. W. Peczuh, A. D. Hamilton, Chem. Rev. 2000, 100, 2479–2494.
- 14For some leading examples of related work involving peptide recognition, see:
- 14aS. R. LaBrenz, J. W. Kelly, J. Am. Chem. Soc. 1995, 117, 1655–1656;
- 14bY. Cheng, T. Suenaga, W. C. Still, J. Am. Chem. Soc. 1996, 118, 1813–1814;
- 14cX. Cha, K. Ariga, T. Kunitake, J. Am. Chem. Soc. 1996, 118, 9545–9551;
- 14dR. Zutshi, J. Franciskovich, M. Shultz, B. Schweitzer, P. Bishop, M. Wilson, J. Chmielewski, J. Am. Chem. Soc. 1997, 119, 4841–4845;
- 14eC. N. Kirsten, T. H. Schrader, J. Am. Chem. Soc. 1997, 119, 12 061–12 068;
- 14fT. D. Clark, J. M. Buriak, K. Kobayashi, M. P. Isler, D. E. McRee, M. R. Ghadiri, J. Am. Chem. Soc. 1998, 120, 8949–8962;
- 14gM. A. Hossain, H.-J. Schneider, J. Am. Chem. Soc. 1998, 120, 11 208–11 209;
- 14hB. Baumeister, S. Matile, Chem. Eur. J. 2000, 6, 1739–1749;
10.1002/(SICI)1521-3765(20000515)6:10<1739::AID-CHEM1739>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar
- 14iY. Takahashi, A. Ueno, H. Mihara, ChemBioChem 2001, 2, 75–79;
- 14jG. Xu, W. Wang, J. T. Groves, M. H. Hecht, Proc. Natl. Acad. Sci. USA 2001, 98, 3652–3657;
- 14kA. Cox, M. M. Arroyo, K. H. Mayo, Biochem. J. 2001, 357, 739–747;
- 14lY. Hamuro, A. D. Hamilton, Bioorg. Med. Chem. 2001, 9, 2355–2363;
- 14mH. Wennemers, M. Conza, M. Nold, P. Krattiger, Chem. Eur. J. 2001, 7, 3342–3347;
10.1002/1521-3765(20010803)7:15<3342::AID-CHEM3342>3.0.CO;2-7 CAS PubMed Web of Science® Google Scholar
- 14nS. T. Phillips, M. Rezac, U. Abel, M. Kossenjans, P. A. Bartlett, J. Am. Chem. Soc. 2002, 124, 58–66;
- 14oH. Zeng, X. Yang, R. A. Flowers, B. Gong, J. Am. Chem. Soc. 2002, 124, 2903–2910;
- 14pK. B. Jensen, T. M. Braxmeier, M. Demarcus, J. G. Frey, J. D. Kilburn, Chem. Eur. J. 2002, 8, 1300–1309.
10.1002/1521-3765(20020315)8:6<1300::AID-CHEM1300>3.0.CO;2-W CAS PubMed Web of Science® Google Scholar
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.