Sex Differences in Associations of Lewy Body Disease with Alzheimer's Disease and Cognitive Decline
Kaitlin B. Casaletto and Jennifer S. Rabin are co-senior authors.
Abstract
Objective
To investigate how sex and age at menopause influence the interplay between Alzheimer's disease (AD) and Lewy body disease (LBD) neuropathologies, and their associations with cognitive decline.
Methods
We analyzed data from: (1) three Rush Alzheimer's Disease Center cohorts (i.e., the Religious Orders Study, Rush Memory and Aging Project, and Minority Aging Research Study), and (2) the National Alzheimer's Coordinating Center Neuropathology Data Set. Neuropathological evaluation assessed LBD (neocortical/limbic-type vs none) and AD, including neuritic plaques (β-amyloid plaques surrounded by dystrophic neurites) and neurofibrillary tangles. In each dataset, we tested interactive associations between LBD and sex on neuritic plaques, neurofibrillary tangles, and cognitive decline. Additionally, in the Rush dataset, we tested whether age at spontaneous menopause modified the associations of LBD with neuritic plaques, neurofibrillary tangles, and cognitive decline in women.
Results
In the Rush dataset, we included 1,277 women and 579 men. In the National Alzheimer's Coordinating Center dataset, we included 3,283 women and 3,563 men. Across both datasets, men were more likely to have LBD, whereas women showed greater neuritic plaque and neurofibrillary tangle burdens. Sex modified the associations of LBD with neurofibrillary tangles (but not neuritic plaques), whereby LBD was more strongly associated with greater neurofibrillary tangle burden in women than men. Men showed faster LBD-related cognitive decline, whereas women showed faster neurofibrillary tangle-related decline, after adjusting for copathologies (neuritic plaques, neurofibrillary tangles, and LBD, as appropriate). In women, earlier age at menopause exacerbated the associations of LBD with neurofibrillary tangle burden and episodic memory decline.
Interpretation
Sex may influence AD and LBD neuropathologies, highlighting the need for precision approaches to dementia prevention and intervention. ANN NEUROL 2025
Alzheimer's disease (AD) and Lewy body disease (LBD) pathologies frequently coexist in the aging brain.1, 2 When examined separately, both AD and LBD pathologies show sex differences. Women show more AD pathology, particularly tau neurofibrillary tangles,3, 4 whereas men show greater LBD (ie, Lewy bodies and Lewy neurites).5, 6 Research on sex differences in the co-occurrence of AD and LBD is limited and has yielded mixed results. One autopsy study found that men were more likely to show pure LBD, whereas the likelihood of co-occurring AD and LBD did not differ by sex.7 By contrast, other autopsy studies of cases with LBD report more severe tau pathology in women than men.8, 9 In vivo studies are also inconsistent. A positron emission tomography study found that compared with men, women diagnosed with the clinical syndrome of dementia with Lewy bodies (DLB) were more likely to have both β-amyloid and tau pathology.10 However, a cerebrospinal fluid study found that women (vs men) with the clinical syndrome of DLB showed a greater burden of β-amyloid, with no sex differences in tau.6 Understanding how sex influences the comanifestation of neuropathologies could inform precision strategies for dementia prevention and treatment.
Sex differences are also evident in clinical syndromes caused by AD and LBD pathologies. Women have nearly double the lifetime risk of AD dementia compared to men.11 Lewy body-related syndromes (eg, Parkinson's disease dementia, DLB) tend to be more common in men,12, 13 although not all studies have shown this sex difference for DLB.14, 15 Although some findings suggest that men are more likely than women to display DLB-associated cognitive and clinical symptoms,16 others have reported the opposite pattern.6, 15 These conflicting findings may reflect heterogeneity in the underlying neuropathologies, highlighting the need for clinicopathological research to clarify sex differences in the cognitive manifestations of LBD and AD.
Sex hormones may contribute to sex differences in LBD, AD, and their co-occurrence.17 Estrogens may provide resistance to neuropathology, with studies linking earlier age at menopause (ie, earlier depletion of ovarian hormones) to greater levels of AD pathology and cognitive impairment.3, 18, 19 Earlier menopause may also increase the risk of Parkinson's disease,20 although findings are limited and inconsistent.21
In the present study, we used 2 independent clinicopathological datasets to comprehensively examine sex differences in the associations between postmortem measures of LBD pathology and AD pathologies, including both neuritic plaques and neurofibrillary tangles, as well as their associations with antemortem cognitive decline. Additionally, we examined whether earlier spontaneous menopause modified the associations of LBD with AD pathologies and cognitive decline.
Materials and Methods
Participants
We used neuropathological and clinical data from 2 sources: (1) 3 Rush Alzheimer's Disease Center cohorts (ie, the Religious Orders Study [ROS],22 the Rush Memory and Aging Project [MAP],22 and the Minority Aging Research Study [MARS]23), and (2) the National Alzheimer's Coordinating Center (NACC) Uniform Data Set24 and Neuropathology Data Set.25 All studies received research ethics approval from relevant Institutional Review Boards, and all participants provided informed consent. We followed the Strengthening the Reporting of Observational Studies in Epidemiology guidelines for cohort studies. We used ROS/MAP/MARS data collected from each study's inception to February 2024, and NACC data from 38 Alzheimer's Disease Research Centers (ADRC) collected between September 2005 and December 2023. There was no overlap in study participants in the datasets.
In both datasets, participants self-reported their sex as female or male, with no data collected on sex at birth versus gender identity. Given the inability to disentangle the effects of biological sex from sociocultural gender, we use “women” and “men” to refer to female and male participants, respectively.
Neuropathology
Neuropathological protocols for ROS/MAP/MARS and NACC have been described previously.25, 26 In ROS/MAP/MARS, brains from decedents were removed and prepared following previously described standardized protocols.26 In NACC, neuropathological examinations were conducted according to the protocols of each ADRC, and data were harmonized according to consensus guidelines.25
LBD
In ROS/MAP/MARS, α-synuclein immunostain (Zymed; 1:50) was used to visualize LBD in 7 brain regions (midfrontal, midtemporal, inferior parietal, anterior cingulate, entorhinal and hippocampal cortices, basal ganglia, and midbrain), as described previously.27 For each participant, LBD was staged as 1 of 4 mutually-exclusive categories: “not present,” “nigral-predominant” (ie, LBD present in the substantia nigra with no evidence of LBD in limbic or neocortical regions), “limbic-type” (ie, LBD present in the anterior cingulate and/or entorhinal regions, with no neocortical LBD as defined as follows), or “neocortical-type” (ie, LBD in either midfrontal, temporal, or inferior parietal cortices, and co-occurring with nigral or limbic LBD).27 Amygdala-predominant LBD is not explicitly recognized in ROS/MAP/MARS. In NACC, LBD was similarly classified as “brainstem-predominant,” “limbic (transitional) or amygdala-predominant”, “neocortical,” or “Lewy bodies present, but region unspecified or found in the olfactory bulb.”
Brainstem- and nigral-predominant LBD alone are rarely associated with DLB clinical and cognitive symptoms,28, 29 and were therefore excluded, along with cases with Lewy bodies in unspecified regions or the olfactory bulb only (excluded: ROS/MAP/MARS: n = 37; NACC: n = 552).
In main analyses, LBD was dichotomized as limbic/neocortical versus absent. We also examined 2 alternative approaches to dichotomizing LBD pathology: (1) limbic/neocortical versus nigral/brainstem-predominant or absent (Table S1), and (2) presence versus absence of any LBD pathology, including nigral/brainstem-predominant, limbic, neocortical, olfactory (NACC only), or unspecified regions (NACC only; Table S2). Both approaches yielded results consistent with the main models (Supplemental Materials; Data S1).
AD
In ROS/MAP/MARS, Bielschowsky silver stain was used to visualize neuritic plaques (β-amyloid plaques surrounded by dystrophic neurites) and tau neurofibrillary tangles in 5 cortical regions (hippocampus and entorhinal, middle temporal, inferior parietal, and middle frontal cortices).30 For each brain region, neuritic plaques and neurofibrillary tangles were counted in the 1-mm2 area of highest density. Summary measures of neuritic plaque (variable name: “plaq_n”) and neurofibrillary tangle (variable name: “nft”) burdens were computed by averaging standardized density counts across the 5 regions. These counts were entered as continuous variables, and were square root transformed before analysis to better approximate normal distributions.31, 32 In NACC, neuritic plaques are staged according to the Consortium to Establish a Registry for Alzheimer's disease (CERAD) score.33 The density of neocortical neuritic plaques is quantified as “none” (C0), “sparse” (C1), “moderate” (C2), or “frequent” (C3). We used the NACC-derived variable “NACCNEUR,” which was constructed to harmonize CERAD scores for neuritic plaques (ie, variable “NPNEUR”) across all versions of the NACC Neuropathology Data Set. Neurofibrillary tangles were assessed according to Braak stage.34 We excluded NACC participants who were not assigned a neurofibrillary tangle Braak stage (ie, non-aging/AD-related; n = 100). For neuritic plaques, in both ROS/MAP/MARS and NACC, quantification was based on cortical density. For neurofibrillary tangles, in ROS/MAP/MARS, quantification was based on tangle density across all available regions, whereas in NACC, it was region-based.
Other Neuropathological Features
Both ROS/MAP/MARS and NACC quantified neuropathological findings related to cerebral amyloid angiopathy (CAA), vascular brain injury, limbic-predominant age-related TDP-43 encephalopathy neuropathologic changes (LATE-NC), and hippocampal sclerosis.7, 25 These measures are detailed in the Supplemental Materials, and were considered in sensitivity analyses.
Cognition
In both ROS/MAP/MARS and NACC, cognition was assessed approximately annually.22, 35 We focused on composite scores of episodic memory, attention/perceptual speed, and executive function (Supplemental Materials; Data S1), which are commonly impaired in DLB.36 In ROS/MAP/MARS, clinical status was assessed annually, and clinical diagnoses of mild cognitive impairment (MCI), AD, PD, and other dementias were made by clinicians.37 DLB diagnoses were not made in ROS/MAP/MARS. In NACC, clinical diagnosis was performed annually for MCI, AD, PD, DLB, and other dementias.35
Menopause History
In ROS/MAP/MARS, women self-reported their age at menopause (“At what age did you stop menstruating?”) and whether menopause was “natural” (ie, spontaneous) or “caused by surgery.” All women were postmenopausal at study entry. No data were collected on whether surgical menopause included oophorectomy, meaning that reported age at menstrual cessation may not reflect age at ovarian failure in women with surgical menopause.38 Therefore, menopause analyses only included women with spontaneous menopause (n = 390 surgical menopause excluded). We additionally excluded women with improbable ages at spontaneous menopause (ie, <20 or >60 years; n = 23).18 Menopause data were not collected in NACC.
APOE Genotype
We categorized participants with 1 or 2 copies of APOE-ε4 as ε4 carriers, and remaining participants as non-carriers. Because many NACC participants were missing APOE data (n = 718), in the main models, we only adjusted for APOE-ε4 carriage (ε4 carrier vs non-carrier) in ROS/MAP/MARS. Sensitivity analyses adjusting for APOE-ε4 in NACC yielded findings consistent with those of the main models (Table S3).
Descriptive Analyses
All analyses were conducted in R (v.4.1.2; The R Foundation for Statistical Computing, Vienna, Austria). We used t tests and χ2 tests to characterize differences in demographic and clinical variables between women and men.
Neuropathology Analyses
All neuropathology analyses adjusted for age at death, APOE-ε4 status (ROS/MAP/MARS only), and copathologies (ie, LBD, neuritic plaques, and neurofibrillary tangles) as appropriate.
Sex Differences in LBD and AD
First, we examined sex differences in the associations between LBD and AD pathologies. In ROS/MAP/MARS, we used logistic and linear regression to assess sex differences in LBD (neocortical/limbic vs none), neurofibrillary tangle burden, and neuritic plaque burden, as appropriate. In NACC, we used logistic and ordinal logistic regression to assess sex differences in LBD (neocortical/limbic vs none), neurofibrillary tangle Braak stage, and CERAD score, as appropriate.
Sex-Specific Associations Between LBD and AD
Next, we evaluated whether sex modified the associations between LBD and AD pathologies. In ROS/MAP/MARS, we used separate linear regression analyses to test interactions between LBD and sex on neuritic plaque and neurofibrillary tangle burdens. In NACC, we used separate ordinal logistic regression analyses to test interactions between LBD and sex on CERAD score and neurofibrillary tangle Braak stage. Due to findings that β-amyloid is more strongly associated with neurofibrillary tangle burden in women than men,4 we adjusted for the interaction between sex and neuritic plaque burden/CERAD score in models where neurofibrillary tangles/neurofibrillary tangle Braak stage was the outcome. Where interactions between sex and LBD were observed, we performed sex-stratified analyses to eludicate associations of LBD with AD in women and men separately.
The Influence of Age at Menopause on Associations Between LBD and AD
Given that sex hormones may contribute to sex differences in LBD, AD, and their co-occurrence,17 we investigated whether age at spontaneous menopause influenced the associations between LBD and neuritic plaques or neurofibrillary tangles. Menopause data were only available in ROS/MAP/MARS. Using linear regression analyses, we examined the interaction between age at menopause and LBD on neuritic plaque and neurofibrillary tangle burdens in separate models.
Cognitive Analyses
Sex-Specific Associations Between LBD and Cognitive Decline
We next investigated sex-specific associations between postmortem LBD and antemortem cognitive decline. To do this, we used linear mixed models with random slopes and intercepts to examine the 3-way interaction between LBD, sex, and time on longitudinal scores of episodic memory, attention/perceptual speed, and executive function. Given that cognition was assessed before autopsy, time was coded as years until death, with death set at time = 0. Because reverse cognitive trajectories were modeled, larger positive beta coefficients reflect faster cognitive decline before death. Although the cognitive data are longitudinal, because cognition was measured before autopsy, these findings do not allow for conclusions about the temporal or causal nature of these associations.
Studies report greater neurofibrillary tangle-related cognitive decline in women compared with men.39 To account for these sex differences, models testing sex-specific associations of LBD with cognitive decline adjusted for the interaction between sex, neurofibrillary tangles/neurofibrillary tangle Braak stage, and time. To control for both baseline age and age at death, which are inherently collinear, we adjusted for the interaction between age at death and number of visits.32 We further adjusted models for years of education, neuritic plaques/CERAD score, APOE-ε4 status (ROS/MAP/MARS only), and their interactions with time, as well as the interval (in years) between the last clinical visit and death. Finally, to adjust for practice effects on neuropsychological tests, we included a term for the square root of the number of previous study visits.40 Where interactions between sex and LBD were observed, we performed sex-stratified analyses to elucidate associations of LBD with cognitive decline in women and men separately.
The Influence of Age at Menopause on Associations Between LBD and Cognitive Decline
In ROS/MAP/MARS, we also tested the 3-way interaction between LBD, age at menopause, and time until death on antemortem cognitive scores. These models adjusted for the interaction between age at death and number of visits, practice effects, and time between last clinical visit and death, along with years of education and its interaction with time, as well as APOE-ε4 status and its interaction with time.
Results with p < 0.05 were considered statistically significant.
Sensitivity Analyses
We performed several sensitivity analyses to assess the robustness of the findings: (1) we adjusted for additional neuropathologies (ie, CAA, hippocampal sclerosis, vascular brain injury, LATE-NC); (2) where possible, we adjusted for clinical diagnoses of AD, PD, and DLB (ie, the final diagnosis available before death); however, DLB diagnoses were not available in ROS/MAP/MARS; (3) we adjusted analyses for cognitive diagnosis at study entry (ie, normal, MCI, or dementia); and (4) given that time is anchored at death, for cognitive models, we removed covariates that relied on information from study entry (ie, the interaction between age at death and number of study visits, practice effects), instead only adjusting for age at death and its interaction with time.
Results
Participant Characteristics
Neuropathological analyses included participants with complete LBD, neuritic plaque, neurofibrillary tangle, and covariate data (ROS/MAP/MARS: n = 1,856; NACC: n = 6,846; Figure S1). Cognitive analyses additionally restricted the sample to participants with at least 2 longitudinal study visits (ROS/MAP/MARS: n = 1,780; NACC: n = 5,089). Table 1 summarizes participant characteristics. In unadjusted χ2 comparisons, in both ROS/MAP/MARS and NACC, men were significantly more likely than women to have LBD. This sex difference was driven by a greater likelihood of neocortical LBD in men than women, although this difference only reached significance in NACC; Table 1).
| Variable | ROS/MAP/MARS | NACC | ||
|---|---|---|---|---|
| Women (n = 1,277, 68.8%) | Men (n = 579, 31.2%) | Women (n = 3,283, 48.0%) | Men (n = 3,563, 52.0%) | |
| Age at baseline (yr), mean (SD) | 80.3 (7.03)* | 79.4 (7.28)* | 76.2 (11.2)* | 73.9 (10.6)* |
| Age at death (yr), mean (SD) | 90.3 (6.65)* | 88.2 (6.62)* | 82.2 (11.7)* | 79.1 (11.1)* |
| Education (yr), mean (SD) | 15.9 (3.37)* | 17.1 (3.98)* | 15.9 (10.2)* | 16.8 (8.54)* |
| Self-reported race/ethnicity | ||||
| White, n (%) | 1,185 (92.8) | 550 (95.0) | 3,026 (92.2)* | 3,397 (95.3)* |
| Black or African American, n (%) | 87 (6.81) | 27 (4.66) | 187 (5.70)* | 97 (2.72)* |
| Asian, n (%) | - | - | 31 (0.94) | 34 (0.95) |
| Othera, n (%) | 5 (0.39) | 2 (0.35) | 39 (1.19) | 35 (0.98) |
| APOE-ε4 carrier, n (%) | 325 (25.5) | 153 (26.4) | - | - |
| Final cognitive diagnosis | ||||
| Normal, n (%) | 426 (33.4) | 192 (33.2) | 541 (16.5)* | 396 (11.1)* |
| MCI, n (%) | 285 (22.3) | 146 (25.2) | 294 (8.96) | 308 (8.64) |
| AD dementia, n (%) | 544 (42.6) | 231 (39.9) | - | - |
| Other (ie, non-AD) dementia, n (%) | 22 (1.72) | 10 (1.73) | - | - |
| Any dementia, n (%) | - | - | 2,448 (74.6)* | 2,859 (80.2)* |
| AD diagnosis, n (%) | - | - | 1,988 (60.6)* | 1,997 (56.0)* |
| PD diagnosis, n (%) | 115 (9.01) | 63 (10.9) | 57 (1.74)* | 150 (4.21)* |
| DLB diagnosis, n (%) | - | - | 119 (3.62)* | 379 (10.6)* |
| LBD pathology | ||||
| Not present, n (%) | 1,017 (79.6)* | 437 (75.5)* | 2,397 (73.0)* | 2,360 (66.2)* |
| Present, n (%) | 260 (20.4)* | 142 (24.5)* | 886 (27.0)* | 1,203 (33.8)* |
| Limbic/amygdala-type, n (%) | 94 (7.36) | 51 (8.81) | 540 (16.4) | 594 (16.7) |
| Neocortical-type, n (%) | 166 (13.0) | 91 (15.7) | 346 (10.5)* | 609 (17.1)* |
| Neuritic plaque score (square root), mean (SD) | 0.79 (0.54)* | 0.65 (0.51)* | - | - |
| Neurofibrillary tangle score (square root), mean (SD) | 0.73 (0.43)* | 0.61 (0.38)* | - | - |
| CERAD neuritic plaque score | ||||
| None (C0), n (%) | - | - | 707 (21.5)* | 858 (24.1)* |
| Mild (C1), n (%) | - | - | 399 (12.2) | 455 (12.8) |
| Moderate (C2), n (%) | - | - | 606 (18.5) | 691 (19.4) |
| Severe (C3), n (%) | - | - | 1,571 (47.9)* | 1,559 (43.8)* |
| Neurofibrillary tangles Braak stage | ||||
| Stage 0, n (%) | - | - | 160 (4.87)* | 247 (6.93)* |
| Stage I, n (%) | - | - | 234 (7.13)* | 310 (8.70)* |
| Stage II, n (%) | - | - | 338 (10.3) | 403 (11.3) |
| Stage III, n (%) | - | - | 358 (10.9) | 357 (10.0) |
| Stage IV, n (%) | - | - | 446 (13.6) | 482 (13.5) |
| Stage V, n (%) | - | - | 573 (17.5) | 654 (18.4) |
| Stage VI, n (%) | - | - | 1,174 (35.8)* | 1,110 (31.2)* |
- * p < 0.05 for t tests and χ2 tests comparing women versus men within each dataset.
- a ROS/MAP/MARS: “Other” includes American Indian or Alaska Native (n = 3 women); Native Hawaiian or Other Pacific Islander (n = 1 woman); and Unknown (n = 1 woman and n = 2 men). NACC: “Other” includes American Indian or Alaska Native (n = 6 women and n = 6 men); Native Hawaiian or Other Pacific Islander (n = 2 women and n = 5 men); Other (n = 14 women and n = 8 men) and Unknown (n = 17 women and n = 16 men).
- AD = Alzheimer's disease; CERAD = Consortium to Establish a Registry for Alzheimer's Disease score for β-amyloid neuritic plaques; DLB = dementia with Lewy bodies; LBD = Lewy body disease; LBD = Lewy body disease; MCI = mild cognitive impairment; PD = Parkinson's disease.
Sex Differences in LBD and AD
In both ROS/MAP/MARS and NACC, men were more likely than women to harbor LBD after adjusting for neuritic plaques and neurofibrillary tangles (ROS/MAP/MARS: OR = 0.684 [95% CI 0.538, 0.872], p = 0.002; NACC: OR = 0.706 [95% CI 0.634, 0.787], p < 0.001). However, women showed higher neuritic plaque burden/CERAD scores than men in both datasets, after adjusting for LBD and neurofibrillary tangles (ROS/MAP/MARS: β = 0.051 [95% CI 0.009, 0.093], p = 0.02; NACC: OR = 1.122 [95% CI 1.010, 1.248], p = 0.03). Women also showed greater neurofibrillary tangle burden/higher neurofibrillary tangle Braak stages compared with men in both datasets after adjusting for LBD and neuritic plaques (ROS/MAP/MARS: β = 0.047 [95% CI 0.014, 0.080], p = 0.005; NACC: OR = 1.191 [95% CI 1.088, 1.304], p < 0.001).
Sex-Specific Associations Between LBD and AD
In both datasets, sex modified the association between LBD and neurofibrillary tangles, but not between LBD and neuritic plaques (Table 2).
| Model | ROS/MAP/MARS | NACC | ||
|---|---|---|---|---|
| β (95% CI) | p | OR (95% CI) | p | |
| Neurofibrillary tangles ~ LBD × female sex + neuritic plaques × female sex + covariates | ||||
| LBD × female sex | 0.102 (0.025, 0.178) | 0.009 | 1.362 (1.112, 1.670) | 0.003 |
| Neuritic plaques × female sex | 0.040 (−0.023, 0.102) | 0.21 | C1: 0.752 (0.561, 1.008) | C1: 0.06 |
| C2: 0.682 (0.522, 0.890) | C2: 0.005 | |||
| C3: 0.760 (0.604, 0.955) | C3: 0.02 | |||
| Neurofibrillary tangles ~ LBD + covariates, women only | ||||
| LBD | 0.107 (0.060, 0.153) | <0.001 | 1.820 (1.558, 2.129) | <0.001 |
| Neurofibrillary tangles ~ LBD + covariates, in men only | ||||
| LBD | −0.002 (−0.059, 0.054) | 0.93 | 1.358 (1.190, 1.549) | <0.001 |
| Neuritic plaques ~ LBD × female sex + covariates | ||||
| LBD × female sex | −0.056 (−0.041, 0.154) | 0.26 | 1.139 (0.900, 1.440) | 0.28 |
| Neurofibrillary tangles ~ LBD × age at menopause + covariates, in women with spontaneous menopause | ||||
| LBD × age at menopause | −0.015 (−0.027, −0.004) | 0.009 | - | - |
| Neurofibrillary tangles ~ LBD + covariates, in women with earlier spontaneous menopause | ||||
| LBD | 0.170 (0.071, 0.269) | <0.001 | - | - |
| Neurofibrillary tangles ~ LBD + covariates, in women with average spontaneous menopause | ||||
| LBD | 0.010 (0.0001, 0.120) | 0.05 | - | - |
| Neurofibrillary tangles ~ LBD + covariates, in women with later spontaneous menopause | ||||
| LBD | 0.007 (−0.093, 0.108) | 0.89 | - | - |
| Neuritic plaques ~ LBD × age at menopause + covariates, in women with spontaneous menopause | ||||
| LBD × age at menopause | 0.004 (−0.011, 0.019) | 0.59 | - | - |
- C1 through C3 = CERAD scores; CI = confidence interval; LBD = Lewy body disease; OR = odds ratio.
ROS/MAP/MARS
LBD was more strongly associated with neurofibrillary tangle burden in women compared to men, adjusting for neuritic plaque burden (Fig 1A). In the same model, the interaction between sex and neuritic plaque burden was not statistically significant. In sex-stratified analyses, LBD was associated with greater neurofibrillary tangle burden in women, but not men.
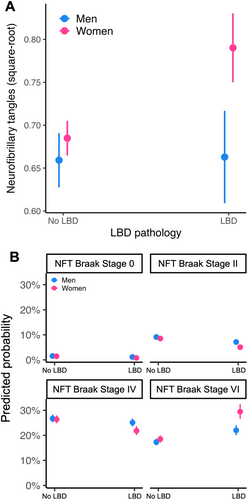
NACC
LBD was more strongly associated with greater neurofibrillary tangle Braak stage in women compared to men, adjusting for CERAD score (Fig 1B). In the same model, the interactions between sex and CERAD scores were also statistically significant. In sex-stratified analyses, LBD was associated with greater neurofibrillary tangle Braak stage in both sexes; however, the effect was almost 2-fold larger in women than in men.
The Influence of Age at Menopause on Associations Between LBD and AD
Menopause analyses included 819 women with spontaneous menopause from ROS/MAP/MARS (mean age at menopause = 48.7 years [SD 5.20 years]). Before examining the influence of age at menopause on associations between LBD and AD, we first tested the main associations between age at menopause and these pathologies. There were no significant associations between age at menopause and LBD (OR = 1.00 [95% CI 0.967, 1.035], p = 0.99), neuritic plaques (β = −0.004 [95% CI −0.009, 0.002], p = 0.19), or neurofibrillary tangles (β = 0.003 [95% CI −0.002, 0.007], p = 0.22).
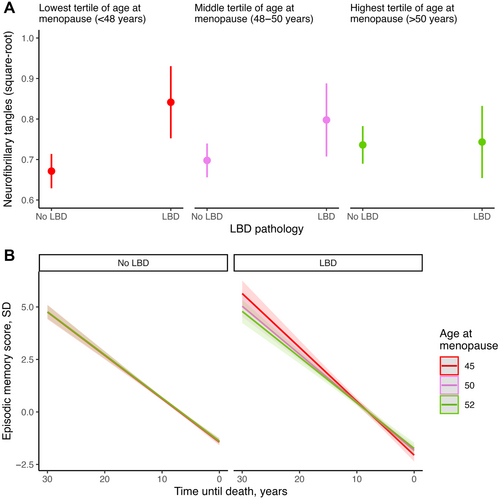
As summarized in Table 2, age at menopause modified the association between LBD and neurofibrillary tangles, but not between LBD and neuritic plaques. Specifically, an earlier age at menopause strengthened the association of LBD with neurofibrillary tangle burden, after adjusting for neuritic plaques. Similar findings were observed when adjusting for menopause hormone therapy (Table S4).
Next, we performed stratified analyses based on age at menopause. The sample was divided into tertiles representing the lowest (<48 years; n = 272), middle (48–50 years; n = 285), and highest (>50 years; n = 262) tertiles of age at menopause. These stratified analyses revealed a dose–response association, such that the magnitude of the association between LBD and neurofibrillary tangle burden increased as age at menopause decreased (Fig 2A). Specifically, LBD was significantly associated with greater neurofibrillary tangle burden in participants with ages at menopause in the lowest and middle tertiles, with the strongest association observed in the former. No significant association was observed among those with age at menopause in the highest tertile (Table 2).
Sex-Specific Associations of LBD with Cognitive Decline
Next, we examined whether sex influenced the associations between LBD and cognitive decline by testing the 3-way interaction between LBD, sex, and time on scores in 3 cognitive domains: episodic memory, attention/perceptual speed, and executive function. These models also included a 3-way interaction between neurofibrillary tangles, sex, and time. Results are presented in Table 3.
| Model | ROS/MAP/MARS | NACC | ||
|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | |
| Episodic memory ~ LBD × female sex × timea + neurofibrillary tangles × female sex × time + covariates | ||||
| LBD × female sex × time | −0.047 (−0.075, −0.020) | 0.001 | −0.010 (−0.028, 0.009) | 0.31 |
| Neurofibrillary tangles × female sex × time | 0.062 (0.032, 0.093) | <0.001 | BI: 0.010 (−0.045, 0.065) | B1: 0.72 |
| BII: −0.027 (−0.078, 0.024) | B2: 0.30 | |||
| BIII: −0.031 (−0.082, 0.019) | B3: 0.23 | |||
| BIV: −0.015 (−0.065, 0.035) | B4: 0.56 | |||
| BV: −0.012 (−0.062, 0.038) | B5: 0.63 | |||
| BVI: −0.010 (−0.059, 0.039) | B6: 0.70 | |||
| Episodic memory ~ LBD × time + covariates, in women only | ||||
| LBD × time | 0.009 (−0.008, 0.026) | 0.29 | - | - |
| Episodic memory ~ LBD × time + covariates, in men only | ||||
| LBD × time | 0.056 (0.034, 0.077) | <0.001 | - | - |
| Attention/perceptual speed ~ LBD × female sex × time + neurofibrillary tangles × female sex × time + covariates | ||||
| LBD × female sex × time | −0.027 (−0.052, −0.003) | 0.03 | −0.036 (−0.054, −0.017) | <0.001 |
| Neurofibrillary tangles × female sex × time | 0.041 (0.013, 0.068) | 0.004 | BI: 0.056 (0.002, 0.111) | B1: 0.04 |
| BII: 0.030 (−0.020, 0.080) | B2: 0.24 | |||
| BIII: 0.013 (−0.037, 0.063) | B3: 0.62 | |||
| BIV: 0.027 (−0.022, 0.076) | B4: 0.28 | |||
| BV: 0.026 (−0.023, 0.074) | B5: 0.30 | |||
| BVI: −0.003 (−0.051, 0.045) | B6: 0.90 | |||
| Attention/perceptual speed ~ LBD × time + covariates, in women only | ||||
| LBD × time | 0.018 (0.003, 0.032) | 0.02 | 0.010 (−0.003, 0.023) | 0.14 |
| Attention/perceptual speed ~ LBD × time + covariates, in men only | ||||
| LBD × time | 0.044 (0.025, 0.064) | <0.001 | 0.046 (0.032, 0.059) | <0.001 |
| Executive function ~ LBD × female sex × time + neurofibrillary tangles × female sex × time + covariates | ||||
| LBD × female sex × time | −0.011 (−0.032, 0.009) | 0.28 | −0.019 (−0.037, −0.002) | 0.03 |
| Neurofibrillary tangles × female sex × time | 0.033 (0.010, 0.056) | 0.004 | BI: 0.033 (−0.019, 0.084) | B1: 0.21 |
| BII: 0.022 (−0.026, 0.069) | B2: 0.37 | |||
| BIII: −0.006 (−0.053, 0.041) | B3: 0.81 | |||
| BIV: −0.009 (−0.056, 0.037) | B4: 0.70 | |||
| BV: −0.0003 (−0.047, 0.046) | B5: 0.99 | |||
| BVI: −0.008 (−0.054, 0.037) | B6: 0.72 | |||
| Executive function ~ LBD × time + covariates, in women only | ||||
| LBD × time | - | - | 0.005 (−0.007, 0.018) | 0.38 |
| Executive function ~ LBD × time + covariates, in men only | ||||
| LBD × time | - | - | 0.023 (0.011, 0.036) | <0.001 |
| Episodic memory ~ LBD × age at menopause × time + covariates, in women with spontaneous menopause | ||||
| LBD × age at menopause × time | −0.005 (−0.010, −0.001) | 0.02 | - | - |
| Attention/perceptual speed ~ LBD × age at menopause × time + covariates, in women with spontaneous menopause | ||||
| LBD × age at menopause × time | −0.001 (−0.005, 0.003) | 0.62 | - | - |
| Executive function ~ LBD × age at menopause × time + covariates, in women with spontaneous menopause | ||||
| LBD × age at menopause × time | 0.0003 (−0.003, 0.003) | 0.84 | - | - |
- a Time is modeled as time until death (in years), such that larger positive coefficients represent steeper cognitive slope; that is, greater antemortem decline.
- AD = Alzheimer's disease; BI through BVI = neurofibrillary tangles Braak stages; C1 through C3 = CERAD scores; CI = confidence interval; LBD = Lewy body disease.
ROS/MAP/MARS
Analyses included n = 1,232 women (mean age at baseline = 80.2 years [SD 7.00 years]) and n = 548 men (mean age at baseline = 79.2 years [SD 7.20 years]). The median number of visits was 9 for women (SD = 5.51) and 8 for men (SD = 5.62).
In terms of episodic memory, we observed a statistically significant interaction between LBD, sex, and time (Figure S2A). In the same model, the interaction between neurofibrillary tangles, sex, and time was also statistically significant. Specifically, LBD was more strongly associated with decline in men, whereas neurofibrillary tangle burden was more strongly associated with decline in women. In sex-stratified analyses, LBD was associated with episodic memory decline in men, but not in women.
There was a statistically significant interaction between LBD, sex, and time on attention/perceptual speed, such that LBD was more strongly associated with decline in men than in women (Figure S2B). The interaction between neurofibrillary tangles, sex, and time was also statistically significant, such that neurofibrillary tangle burden was more strongly associated with decline in women relative to men. In sex-stratified analyses, LBD was associated with attention/perceptual speed decline in both men and women, but the magnitude of the effect was more than twice as large in men.
Regarding executive function, the interaction between LBD, sex, and time was not statistically significant (Figure S2C). However, as with other domains, the interaction between neurofibrillary tangles, sex, and time was statistically significant, such that neurofibrillary tangle burden was more strongly associated with cognitive decline in women than men.
NACC
Analyses included n = 2,455 women (mean age at baseline = 76.3 years [SD 10.5 years]) and n = 2,634 men (mean age at baseline = 74.1 years [SD 10.2 years]). The median number of visits was 5 for women (SD = 2.96) and 4 for men (SD = 2.72).
For episodic memory, the interaction between LBD, sex, and time was not statistically significant (Figure S3A), nor were the interactions between neurofibrillary tangle Braak stages, sex, and time.
Regarding attention/perceptual speed, there was a statistically significant interaction between LBD, sex, and time, such that LBD was more strongly associated with decline in men than in women (Figure S3B). Sex-stratified analyses showed that LBD was associated with attention/perceptual speed decline in men, but not women. The interactions of neurofibrillary tangle Braak stages, sex, and time were not statistically significant, except for Braak stage I, whereby stage I (vs stage 0) was more strongly associated with decline in women than in men.
For executive function, the interaction between LBD, sex, and time was statistically significant, such that LBD was more strongly related to decline in men than in women (Figure S3C). The interactions between neurofibrillary tangle Braak stages, sex, and time were not statistically significant. In sex-stratified analyses, LBD was associated with executive function decline in men, but not in women.
The Influence of Age at Menopause on Associations Between LBD and Cognitive Decline
These analyses included 795 women with spontaneous menopause (mean age at menopause = 48.7 years [SD = 5.25 years]) from ROS/MAP/MARS. There was a statistically significant interaction between LBD, age at menopause, and time on episodic memory (Fig 2B), whereby LBD was more strongly associated with decline in women with earlier menopause compared to women with later menopause. The same interactions were not statistically significant for attention/perceptual speed or executive function. Adjusting for menopause hormone therapy in these analyses yielded similar findings (Table S4).
Sensitivity Analyses
First, we examined whether adjusting for additional neuropathologies, including CAA, hippocampal sclerosis, vascular brain injury, and LATE-NC, influenced our results. Descriptive statistics for these non-AD and non-LBD pathologies are presented in Table S5. These analyses showed that the prevalence of CAA, hippocampal sclerosis, and vascular brain injury did not significantly differ by sex. However, women were more likely than men to show LATE-NC pathology (available in ROS/MAP/MARS only) in its most advanced stage (ie, present in amygdala, limbic, and neocortical regions). Sensitivity analyses adjusting for these pathologies yielded results consistent with the main models (Table S6). Additional sensitivity analyses were also consistent with the main models, suggesting they were not driven by clinical phenotypes (Table S7), cognitive diagnosis at study entry (Table S8), or temporal biases (Table S9).
Discussion
Across 2 large clinicopathological datasets, we examined whether sex differentially influenced the associations among LBD, AD, and cognitive decline. Consistent with previous reports,3-5, 7 men were more likely to have LBD, whereas women showed greater neuritic plaque and neurofibrillary tangle burdens. There were 3 novel findings: (1) LBD was associated with disproportionately greater neurofibrillary tangle burden in women than in men; (2) men showed greater cognitive decline in the presence of LBD, whereas women showed greater cognitive decline in the presence of neurofibrillary tangles; and (3) women with earlier (vs later) spontaneous menopause showed a stronger association between LBD and greater neurofibrillary tangle burden, as well as between LBD and faster episodic memory decline. These findings build on emerging evidence that sex plays a fundamental role in the manifestation of neurodegenerative pathologies, underscoring the need for disease-modifying trials to carefully consider sex differences.
LBD was more strongly associated with greater neurofibrillary tangle burden in women than in men in both ROS/MAP/MARS and NACC, aligning with previous research showing that co-occurring LBD and AD pathologies are more prevalent in women.8-10 Our findings further suggest that these sex-specific effects may not simply reflect a broader female susceptibility to AD. Instead, the presence of LBD may uniquely increase the risk for neurofibrillary tangles (but not neuritic plaques) in women, but not in men. These data build on recent findings that women have higher tau levels than men at comparable levels of β-amyloid, further supporting a female-specific susceptibility to neurofibrillary tangle aggregation (and not neuritic plaques) in the presence of other proteinopathies.4 Notably, in this study, the sex-specific association of LBD with neurofibrillary tangle burden was stronger than that of the previously established sex-specific association between neuritic plaque and neurofibrillary tangle burdens.
In men, LBD was not only more prevalent, but also more strongly associated with cognitive decline, although the specific cognitive domains varied somewhat across datasets. The finding that LBD was more strongly associated with decline in attention/perceptual speed in men than in women was consistent across both ROS/MAP/MARS and NACC. We also observed greater male vulnerability to LBD-related decline in episodic memory in ROS/MAP/MARS, and executive function in NACC. These discrepancies may reflect that different cognitive tests were used to construct the domain-specific composite scores in each dataset, which may capture distinct aspects of the cognitive domains and/or differ in their psychometric properties.
In the literature, findings on sex-specific associations of LBD with clinical symptoms are mixed.6, 15, 16 These inconsistencies may relate to the specific cognitive tests used across studies, but more critically, to whether analyses account for co-occurring AD pathology. For instance, 1 study reported worse cognition in women compared with men with DLB in analyses that did not adjust for AD pathology.6 In contrast, studies that account for AD pathology,16 including the present study, report greater LBD-related cognitive decline in men compared with women. Our findings on neurofibrillary tangle-related cognitive decline are consistent with prior research,39 showing stronger effects in women relative to men. However, this pattern was observed only in ROS/MAP/MARS and not in NACC. This discrepancy may reflect differences in how neurofibrillary tangle pathology was measured (ie, neurofibrillary tangle density score vs neurofibrillary tangle Braak Stage).
Interestingly, we found that women who experienced earlier (vs later) spontaneous menopause showed a stronger association between LBD and neurofibrillary tangle burden. They also showed a stronger link between LBD and episodic memory decline, likely driven in part by the greater neurofibrillary tangle burden in women with both LBD and earlier menopause. These results, together with the observed sex differences, suggest that ovarian hormone depletion due to menopause may play a key role in these relationships. Estrogens are neuroprotective, and menopause-related estradiol depletion may increase the brain's susceptibility to neuropathology and cognitive decline.18, 19, 41-43 Although the underlying mechanisms remain incompletely understood, our results suggest that this heightened vulnerability may also influence the interactions between LBD and neurofibrillary tangles. Future research should explore the menopause transition as a critical window for understanding how hormonal changes influence neuropathological burden.
In this study, we hypothesized that LBD precedes and exacerbates neurofibrillary tangle pathology, given evidence suggesting that α-synuclein, the primary component of Lewy bodies, may accelerate the spread of tau pathology.44-46 However, our study design did not allow us to determine the temporality of the association between LBD and neurofibrillary tangles. It is possible that in women, LBD is primarily a consequence of advanced AD pathology, whereas in men, LBD may occur independently of AD pathology. Another possibility is that both LBD and neurofibrillary tangles are driven by a shared upstream process, such as β-amyloid accumulation. This concept is supported by research in familial AD and Down syndrome,47, 48 where LBD frequently co-occurs with AD, particularly in younger populations who would not typically present with LBD in the absence of AD.
A strength of this study was the replication of sex-specific effects of LBD across 2 large datasets, despite differences in recruitment, demographics, and neuropathological assessments. There were also limitations. First, the categorical measures of LBD precluded us from evaluating dose–response effects of cortical LBD. Second, because neuropathological measures were quantified postmortem, we could not assess the temporal dynamics of the reported associations. As in previous studies,32, 49 we made assumptions about the temporal sequencing of events, namely that cognitive decline results from neuropathology—despite the fact that neuropathology was measured after cognitive assessments. Third, methodological differences in pathological assessments exist both among NACC sites and between NACC and ROS/MAP/MARS. However, the established reliability of AD measures across NACC centers partially mitigates this limitation.50 Finally, differences in study design and recruitment practices may introduce selection biases: NACC primarily includes clinical participants, whereas ROS/MAP/MARS recruits older, community-dwelling participants. This may partly explain the substantially higher proportion of women participants in ROS/MAP/MARS compared with NACC.
To conclude, this study demonstrated that sex influences AD and LBD pathologies, as well as their comanifestation, which in turn affects cognitive outcomes. These sex differences may be driven by sex-specific hormonal processes. Understanding sex differences in neuropathological patterns is crucial for developing precision approaches to prevent and treat neurodegenerative conditions.
Acknowledgment
The NACC database is funded by National Institute on Aging/National Institutes of Health Grant U24 AG072122. NACC data are contributed by the NIA-funded ADRCs: P30 AG062429 (PI James Brewer, MD, PhD), P30 AG066468 (PI Oscar Lopez, MD), P30 AG062421 (PI Bradley Hyman, MD, PhD), P30 AG066509 (PI Thomas Grabowski, MD), P30 AG066514 (PI Mary Sano, PhD), P30 AG066530 (PI Helena Chui, MD), P30 AG066507 (PI Marilyn Albert, PhD), P30 AG066444 (PI John Morris, MD), P30 AG066518 (PI Jeffrey Kaye, MD), P30 AG066512 (PI Thomas Wisniewski, MD), P30 AG066462 (PI Scott Small, MD), P30 AG072979 (PI David Wolk, MD), P30 AG072972 (PI Charles DeCarli, MD), P30 AG072976 (PI Andrew Saykin, PsyD), P30 AG072975 (PI Julie Schneider, MD), P30 AG072978 (PI Neil Kowall, MD), P30 AG072977 (PI Robert Vassar, PhD), P30 AG066519 (PI Frank LaFerla, PhD), P30 AG062677 (PI Ronald Petersen, MD, PhD), P30 AG079280 (PI Eric Reiman, MD), P30 AG062422 (PI Gil Rabinovici, MD), P30 AG066511 (PI Allan Levey, MD, PhD), P30 AG072946 (PI Linda Van Eldik, PhD), P30 AG062715 (PI Sanjay Asthana, MD, FRCP), P30 AG072973 (PI Russell Swerdlow, MD), P30 AG066506 (PI Todd Golde, MD, PhD), P30 AG066508 (PI Stephen Strittmatter, MD, PhD), P30 AG066515 (PI Victor Henderson, MD, MS), P30 AG072947 (PI Suzanne Craft, PhD), P30 AG072931 (PI Henry Paulson, MD, PhD), P30 AG066546 (PI Sudha Seshadri, MD), P20 AG068024 (PI Erik Roberson, MD, PhD), P20 AG068053 (PI Justin Miller, PhD), P20 AG068077 (PI Gary Rosenberg, MD), P20 AG068082 (PI Angela Jefferson, PhD), P30 AG072958 (PI Heather Whitson, MD), and P30 AG072959 (PI James Leverenz, MD). MAP/ROS/MARS are funded by National Institute on Aging/National Institutes of Health grants P30AG072975, P30AG010161, R01AG017917, and R01AG022018.
Author Contributions
M.W.A., D.L.F., L.V., K.B.C., and J.S.R. contributed to the conception and design of the study; M.W.A., K.B.C., and J.S.R. contributed to the acquisition and analysis of data; M.W.A., K.B.C., and J.S.R. contributed to drafting the text or preparing the figures. All authors contributed to the review and editing of the manuscript.
Potential Conflicts of Interest
Nothing to report.
Open Research
Data Availability
Data availability for ROS/MAP/MARS and NACC can be requested by qualified investigators.




