Mapping Postictal Aphasia through Signal Complexity: A Stereo-Electroencephalography Study
Fabrice Bartolomei and Agnès Trébuchon contributed equally to this work.
Abstract
Objective
The postictal period provides an opportunity to investigate the pathophysiology underlying aphasia and recovery following epileptic seizures. This study examines postictal aphasia in stereo-electroencephalography (SEEG)-explored patients to identify brain regions associated with task-specific language deficits using signal complexity analysis.
Methods
We evaluated video-SEEG-recorded focal seizures with and without postictal aphasia in patients with SEEG-confirmed hemispheric language dominance. SEEG traces were analyzed using permutation entropy (PE), with the postictal period quantified by a PE-based metric, the Postictal Alteration Time (PAT). Brain region PAT was correlated with language function recovery (eg, repetition). Electro-clinical recuperation was also assessed within the dorsal-ventral language stream framework. Additionally, a bedside testing battery was developed to evaluate postictal aphasia severity and task-specific deficits.
Results
A total of 322 seizures from 98 patients were analyzed. Seizures with postictal aphasia had longer PAT than those without. Task-specific language recovery correlated with regional PAT (eg, naming – middle temporal gyrus). Moreover, the dorsal stream recovered faster than the ventral stream. Additionally, the Postictal Aphasia Scale (PAS) was developed, evaluating naming, reading, repetition, and comprehension (verbal and written) and automatic speech. Higher PAS scores (indicating milder deficits) correlated with faster regional complexity recovery. At 5 and 10 minutes postictally, PAS revealed a global aphasia pattern, with comprehension deficits gradually resolving. By 15 minutes, aphasia was primarily production-related, particularly affecting naming.
Interpretation
This study provides new insights into the pathophysiology of postictal aphasia and introduces PAS as a tool for assessing postictal aphasia severity and domain-specific deficits, aiding surgical planning and rehabilitation. ANN NEUROL 2025
Epilepsy surgery aims to achieve seizure freedom with minimal postoperative deficits. In this context, presurgical evaluation is essential, involving an interdisciplinary approach to identify and map the epileptogenic-zone network (EZN), the eloquent cortex and to understand their relationship.1 Whereas primary motor and sensory areas have relatively predictable localization and can often be easily identified and mapped, the cerebral organization of language processing is far more complex and variable.2 Traditional models, such as the Broca-Wernicke-Geschwind, are now understood to oversimplify the cortical organization of language.3 More recent models propose a dual-stream framework, with a dorsal stream involving phonological processing and speech production and a ventral stream being responsible for speech comprehension and semantic processing.4 However, interindividual variability in cerebral language processing organization further complicates presurgical mapping, especially in patients with atypical brain anatomy due to congenital lesions or long-standing epilepsy, where functional reorganization may have occurred.5 Several methods are used during presurgical language evaluation (eg, functional magnetic resonance imaging [fMRI] and WADA testing). Nonetheless, electrophysiological approaches, particularly direct electrical cortical stimulation, remain the gold standard for language mapping.6 Such assessments can be performed intraoperatively or during chronic invasive electroencephalogram (EEG) monitoring and involve stimulating different cortical regions while the patient performs language tasks. Disruption of language functions during stimulation suggests that the stimulated region or sites with preferential connectivity to that region may be critical for the specific language task being performed. Moreover, language deficits during or after seizures can also provide important information regarding the lateralization and localization of language networks. However, the postictal phase has been understudied in the context of presurgical language mapping. The postictal state refers to an abnormal cerebral condition following a seizure, during which the brain recovers to its baseline function. The postictal period is often characterized by neurological deficits that can last well beyond the “normalization” of the EEG trace.7 Postictal aphasia, a transient inability to use and/or understand language, occurs when seizures involve language processing brain regions and affects approximately 30% of patients.8
Given the nonlinear dynamics of brain activity, methods such as permutation entropy (PE) have demonstrated reliability in analyzing stereo-electroencephalographic (SEEG) signals.9 Distinctive SEEG entropy signal dynamics have permitted the quantification of the postictal period and its association with contrasting behavioral disturbances (asthenia and aggression) and clinical deficits (memory).10, 11
The current study investigated postictal aphasia using an electro-anatomic-clinical approach in SEEG-explored patients. Our first objective was to identify key brain regions involved in the pathophysiology of deficits affecting distinct language abilities. To achieve this, we sought to study the relationship between the recovery of signal complexity in SEEG-explored brain regions, as measured by PE, and the recovery of different language abilities affected during postictal aphasia. Moreover, starting from these regional-level associations, we aimed to assess the electro-anatomic-clinical recovery considering the dual-stream language framework. The second objective was to develop an easy-to-use bedside scale for quantifying postictal aphasia and explore its relevance in light of the brain regional complexity recovery.
Methods
Patient Selection and Data Acquisition
Patients were selected from the Epileptology database of Timone Hospital, Marseille, if they had: video-SEEG-recorded seizures with testing-proven postictal aphasia, pre/post-implantation cerebral computed tomography-magnetic resonance imaging (CT-MRI) data and SEEG confirmation of hemispheric language dominance.
Presurgical evaluations included detailed medical history, neurological examination, neurocognitive testing, cerebral fluorodeoxyglucose-positron emission tomography (FDG-PET) and MRI scans and long-term scalp-video-EEG and SEEG monitoring. Additional assessments (eg, language fMRI, Wada testing, cerebral 7T MRI, and magnetoencephalography [MEG]) were conducted on a case-by-case basis. Demographic, clinical, and follow-up data were retrieved from medical records. All patients provided informed written consent and the study was approved by the ethics committee of Assistance Publique – Hôpitaux de Marseille (PADS VNMG2V2024).
SEEG Procedure
SEEG explorations were conducted in accordance with the French national guidelines,12 using 10 to 18-contact intracerebral electrodes (Dixi or Alcis; 2 mm contact length, 1.5 mm spacing, and 0.8 mm diameter) implanted based on the noninvasive anatomic-electro-clinical EZN hypothesis. Post-implantation cerebral CT and/or MRI scans were conducted to verify electrode placement accuracy and identify any periprocedural complications. Signal recording was performed on a 128 or 256-channel Natus system, with a sampling rate of 512 or 1,024 hertz (Hz). Hardware filtering included a high-pass filter (1 Hz cutoff, −3 decibels [dB]) and an anti-aliasing low-pass filter (170 Hz or 340 Hz cutoff ).
SEEG Signal Analysis
All signal analyses were performed in AnyWave software13 in bipolar montage. Any channels showing artifacts were excluded based on visual inspection. The EZN, the propagation-zone network (PZN) and the non-involved areas (NIZ) were defined by 3 expert clinicians (authors I.F.B., F.B., and A.T.), based on visual and quantitative SEEG-signal analysis using the Epileptogenicity Index (EI) and the Connectivity Epileptogenicity Index (cEI).14, 15 The EI measures epileptogenicity based on the energy ratio between high-frequency and low-frequency bands and the delay of the abrupt frequency transition in a specific brain structure relative to the first structure involved by the fast discharge. The cEI integrates the EI with a measure of directed functional connectivity (“out-degree”), allowing the evaluation of the leading nodes in the network.
Permutation Entropy Method
PE quantifies the probability distribution of ordinal patterns, which are determined by the rank of values sampled at equidistant points in a time series.16 Starting with a one-dimensional time series, vectors are created based on the embedding dimension D, which determines the number of samples per vector and a fixed time delay τ, for sample selection. Each vector is assigned an ordinal pattern π, corresponding to the rank of sample amplitudes. The probability p(π) of each ordinal pattern occurring within a given time window is then computed. The entropy within the window is defined as . The values of H(D) range from 0 to 1, where smaller values indicate a more regular and deterministic time series and values closer to 1 indicate a noisier, more random time series. SEEG PE analysis was performed using an in-house Matlab (Mathworks, Natick, MA) plug-in (details in El Youssef et al).17 Based on exploratory testing, review of the literature, and our previous work, the PE analysis parameters used were D = 3 samples and τ = 1 sample (consecutive observations), using a 5-second sliding time window with a 2.5-second overlap.9, 10, 18
Definition of Regions of Interest
To accurately map SEEG contacts to brain regions, GARDEL software was used.19 It co-registers the pre-implantation cerebral T1-MRI with the post-implantation cerebral CT images, followed by semi-automatic recognition and anatomic localization of each electrode contact, based on the Virtual Epileptic Patient (VEP) atlas20 parcellation of the patient's cerebral MRI scan.
Network and State Assessment
A thorough visual analysis of (peri)ictal SEEG recordings was performed. Seizure onset was defined as the first SEEG change within a sustained rhythmic discharge and seizure offset as the cessation of ictal activity in the last involved bipolar derivation. Contacts were classified as EZN (EI ≥ 0.4 and/or cEI ≥ 0.65), PZN (0.1 < EI < 0.4 and/or 0.3 < cEI < 0.65) or NIZ (all other contacts).21 False cEI positives values, such as those arising from physiological fast activity (eg, motor cortex sampling or during cortical arousal), were identified and excluded from cEI computation following expert visual inspection. Comprehensive clinical assessment and SEEG trace inspection were critical for final classification, beyond quantified biomarkers. Brain regions were classified using the maximal cEI value when sampled by multiple contacts.
PE values were computed for the (peri)ictal periods of spontaneous and stimulation-induced seizures. As per our previous study,10 we considered that the SEEG signal returned to its preictal complexity (“come-back point”) when for 25 consecutive seconds onward (10 × 2.5-second overlap) the mean PE value returned within the preictal 2 SD baseline interval. The period between seizure offset and the come-back point was named Postictal Alteration Time (PAT). The PAT was quantified globally (mean across all channels; Fig 1A), hemispherically (mean per hemisphere; Fig 1B) and regionally (maximum PAT value from all channels sampling a specific cerebral area).
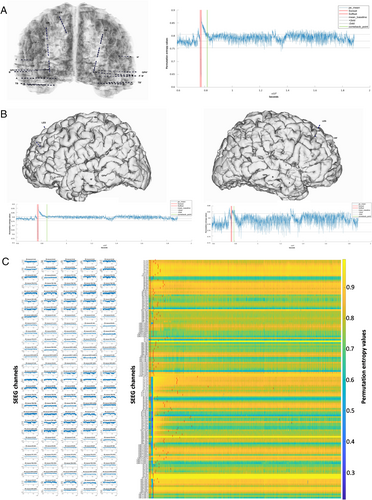
Language Organization Exploration
Hemispheric language dominance was determined on a case-by-case basis using both noninvasive and invasive assessments.6 The noninvasive explorations included: (1) video-EEG electro-clinical ictal and postictal correlations, (2) clinical data (eg, handedness), (3) age-appropriate neuropsychological testing (eg, the Wechsler Adult Intelligence Scale and the Wechsler Intelligence Scale for Children), and (4) language fMRI. Invasive assessments involved: (1) video-SEEG electro-clinical correlations during ictal and postictal periods, (2) SEEG recording of auditory cortex evoked potentials in response to French-language voiced (/ba/) and voiceless (/pa/) stop consonants, (3) functional mapping of the language eloquent cortex using direct electrical cortical stimulations and identification of regions producing gamma activity during a picture naming task, and (4) WADA testing.
Postictal Aphasia Bedside Evaluation
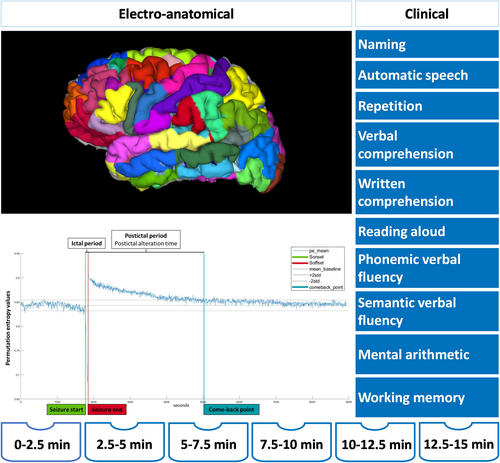
Postictal language assessment was performed at the time of the video-SEEG exploration using an in-house test battery (Supplementary Fig S1). It addressed the following tasks: naming, automatic speech (eg, reciting days/months/seasons or counting), repetition (eg, 2 words of varying complexity and a complete phrase), verbal and written comprehension (eg, simple and complex commands), reading aloud (eg, simple words, phrases and logatomes), phonemic and semantic verbal fluency (eg, words starting with “L” and naming fruits), and working memory (eg, 3-word recall and simple mental arithmetic).
Electro-Anatomic-Clinical Postictal Aphasia Analysis
Video-SEEG clinical and electrical data were first analyzed separately and independently. Postictal clinical language evaluations were retrospectively carried out by author Ionuț-Flavius Bratu using ictal-postictal video-SEEG recordings. As per standard practice in our department, patients underwent postictal language testing within 3 defined time intervals: 0 to 5, 5 to 10, and 10 to 15 minutes after seizure termination. For finer electro-anatomic-clinical correlations, each tested language domain was assigned to a 2.5-minute subinterval within the 0 to 15 minutes postictal window (0–2.5, 2.5–5, 5–7.5, 7.5–10, 10–12.5, and 12.5–15 minutes; Fig 2). Each language domain was scored as 0 (no recovery), 1 (partial recovery), or 2 (full recovery). Postictal brain region complexity recovery (PAT per region) was quantified according to the previously described considerations and classified according to the 6 postictal time intervals.
To assess the relationship between postictal language domain recovery and entropy recuperation in distinct left-hemispheric regions of SEEG-confirmed left-hemisphere language-dominant patients (see Fig 2), we used a comprehensive approach that included region selection based on frequency of occurrence across seizures (eg, due to SEEG-sampling), statistical resampling and permutation testing. Brain regions sampled in at least 12 seizures were retained, even if all 12 seizures came from a single patient, to balance statistical power with adequate representation of less frequently sampled areas.
Co-occurrences of the entropy and language domain recovery for each brain region were computed across the six 2.5-minute intervals. We applied a permutation-based resampling method to assess the significance of observed co-occurrences. In each of 1,000 permutations, shuffling was performed at multiple levels: first, the language recovery intervals were randomly shuffled within each patient, then within each seizure, and finally the patients were shuffled as an “en bloc” group (stratified by the number of seizures). This generated a null distribution of match probabilities between shuffled language recovery intervals and the original entropy recovery intervals. From this null distribution, a 95th percentile threshold was derived as the cutoff for statistical significance. The observed proportion of co-occurrences, defined as the fraction of matching intervals between language recovery and entropy recovery, was evaluated against this threshold. Regions where the observed proportion exceeded the threshold were considered to show statistically significant electro-clinical correlations, entropy-language domain recovery.
Ventral-Dorsal Stream Electro-Clinical Framework
Stream dichotomization was assessed from an electrical-clinical point of view: stream PAT recovery and language recuperation. Using the VEP atlas parcellation, we classified regions in ventral and/or dorsal language stream areas based on a literature review3, 22 (Supplementary Table S1). For each stream, PAT was computed from the highest values of the individual brain regions. We further analyzed a subgroup of seizures in which 3 specific language abilities were tested in the same 2.5-minute interval at the beginning of the assessment and whose recovery could be traced reliably across the intervals: (1) reading aloud (reflecting both streams, dorsal more than ventral), (2) written comprehension (for the ventral stream), and (3) repetition (for the dorsal stream). This analysis was carried out in left hemisphere regions in SEEG-confirmed left-hemisphere language-dominant patients.
Postictal Aphasia Scale
To assess inter-rater agreement, postictal language evaluations were performed on a subset of seizures by 2 assessors (author I.F.B. and the speech therapy team: authors L.S. and F.M.), using the same scoring criteria as previously described. Language performance across relevant domains was evaluated at 2.5-minute intervals during the postictal period, based solely on bedside video recordings. Although author Ionuț-Flavius Bratu was aware of the inclusion criteria for patient selection, all video analyses were performed blinded to other clinical and electrophysiological information. The second rater, independently re-scored the same seizures, fully blinded to electro-clinical data. Inter-rater agreement was evaluated using the quadratic weighted kappa test, which assigns higher penalties for larger rating discrepancies. Language functions that were frequently tested and showed high bedside feasibility and agreement were selected to create a simplified postictal aphasia bedside scale. This scale was applied to re-analyze data at 5-minute intervals and its scores were assessed in relationship to left-hemisphere regional PAT in SEEG-confirmed left-hemisphere language-dominant patients.
Statistical Analysis
Statistical analyses were conducted using GraphPad Prism (version 10.3.1), MedCalc Software (version 22.026), Matlab (version 2023b), and RStudio (version 4.3.2) for Windows. The Shapiro–Wilk normality test guided the choice of parametric versus nonparametric tests (threshold p < 0.05). To address multiple comparisons across several brain regions, the false discovery rate (FDR) correction was applied (Benjamini–Hochberg Method), Matlab (version 2023b) and RStudio (version 4.3.2) for Windows.
For patients who experienced both seizure types (with and without postictal aphasia), PAT values were averaged per seizure type and compared using the Wilcoxon signed-rank test. Furthermore, linear mixed-effects models were fitted using the lmerTest package in R software to account for repeated measures in patients with multiple seizures and relevant demographic and clinical covariates. The outcome variable was PAT (global or at dominant hemisphere level). Postictal aphasia (present/absent) was included as the main fixed effect of interest, with seizure type (spontaneous/stimulation-induced), age group (pediatric = < 18 years old and adults = ≥ 18 years old) and EZN-language overlap (complete, partial, or none) as additional covariates. A random intercept accounted for repeated seizures per patient. Similar models were applied to study the Postictal Aphasia Score (PAS) score (at different postictal time points) as the outcome variable and regional PAT as the main fixed effect, controlling for the same covariates. For network-level PAT analysis, the maximum PAT per region was first identified, then averaged across the regions within each network (EZN, PZN, or NIZ) and across seizures for each patient. For comparisons between PAT values at the network level, we used Kruskal-Wallis test with post hoc correction (Dunn's test). To account for repeated measures (potentially several seizures per patients), a linear mixed-effects model was also fitted with the network as a fixed effect and the patient as random effect.
Results
Cohort Characteristics
Patients’ characteristics are summarized in Table 1 and individual patient-specific information are provided in Supplementary Table S2. We analyzed 322 focal seizures (without bilateral to tonic–clonic evolution) from 98 patients. Postictal aphasia occurred in 237 seizures (31 stimulation-induced and 206 spontaneous). Eighty-five seizures were not followed by aphasia (25 stimulation-induced and 60 spontaneous). The mean age at SEEG was 32.21 years (range = 6–61 years). Seventy percent of the cases had temporal EZN, 18% were temporal plus, and 12% were extra-temporal. EZN was left-sided in 60% of patients, right-sided in 16%, and bilateral in 24%. Post-SEEG curative-aim epilepsy surgery was performed in 52% of patients, with an Engel I outcome at last follow-up achieved in 63%. The most common histopathological finding was focal cortical dysplasia (25%), whereas 29% of the resected tissue samples showed no specific pathology. SEEG language assessment revealed typical left hemisphere language organization in 84 patients (280 seizures), atypical bilateral organization in 12 cases, and atypical right hemisphere organization in 2 cases.
| Sex, M/F, %, no. | 42/58 (41/57) |
| Age at epilepsy onset, yr, mean ± SD (range) | 16.44 ± 10.50 (0–47) |
| Age at SEEG, yr, mean ± SD (range) | 32.21 ± 13.17 (6–61) |
| Epilepsy duration until SEEG, yr, mean ± SD (range) | 15.77 ± 10.96 (1–46) |
| Epileptogenic-zone network type, %, no. | |
| Temporal | 70 (69) |
| Lateral | 9 (9) |
| Mesial | 39 (40) |
| Lateral-mesial | 7 (7) |
| Temporal-plus | 18 (18) |
| Temporal-frontal | 4 (4) |
| Temporal-insular | 4 (4) |
| Temporal–parietal | 1 (1) |
| Temporal-occipital | 1 (1) |
| Temporal-frontal-insular | 3 (3) |
| Temporal-frontal–parietal | 1 (1) |
| Temporal–parietal-insular | 1 (1) |
| Temporal–parietal-occipital | 3 (3) |
| Extra-temporal | 12 (11) |
| Frontal | 6 (6) |
| Insular-frontal | 1 (1) |
| Insular-parietal | 3 (3) |
| Parietal | 1 (1) |
| Epileptogenic-zone network lateralization, %, n | |
| Left | 60 (59) |
| Right | 16 (16) |
| Bilateral | 24 (23) |
| SEEG language testing hemispheric dominance, %, no. | |
| Typical-left | 86 (84) |
| Atypical-right | 2 (2) |
| Atypical-bilateral | 12 (12) |
| Post-SEEG curative-aim epilepsy surgery, %, no. | |
| Yes | 52 (51) |
| Resection | 92 (47) |
| Gamma knife | 6 (3) |
| Laser interstitial thermal therapy | 2 (1) |
| No | 48 (47) |
| Histopathology, % | |
| Focal cortical dysplasia | 25 (12) |
| Hippocampal sclerosis | 23 (11) |
| Cavernoma | 1 (1) |
| Low-grade epilepsy-associated neuroepithelial tumors | 14 (7) |
| Gliosis | 6 (3) |
| Non-specific | 29 (14) |
| Not available | 2 (1) |
| Outcome at last follow-up (Engel class), %, no. | |
| I | 63 (32) |
| Non-I | 37 (19) |
- No. = number; SD = standard deviation; SEEG = stereo-electroencephalography.
Postictal Electro-Clinical Correlations
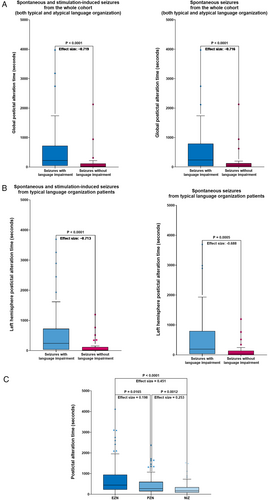
Global PAT was statistically significantly higher in seizures followed by postictal deficit compared with those without postictal aphasia (Wilcoxon signed-rank test, p < 0.0001), both in the entire seizure cohort (spontaneous and stimulation-induced, effect size −0.719) and for spontaneous seizures alone (effect size = −0.716; Fig 3A). This was also true for left hemisphere PAT in patients with SEEG-confirmed left-hemispheric language dominance (Wilcoxon signed-rank test, p < 0.001; spontaneous and stimulation-induced seizures, effect size = −0.713 and spontaneous seizures alone, effect size = −0.688; Fig 3B). Furthermore, PAT revealed a hierarchical distinction among networks: EZN > PZN > NIZ (Fig 3C), with a longer PAT in more epileptogenic regions (Kruskal-Wallis analysis with post hoc Dunn's test; EZN vs. NIZ, p < 0.0001, effect size = 0.451; EZN vs. PZN, p = 0.0165, effect size = 0.198 and PZN vs. NIZ, p = 0.0012, effect size = 0.253). These results were confirmed (p < 0.05) by mixed-effects models accounting for both repeated measurements for patients with multiple seizures and relevant patient demographic and clinical features.
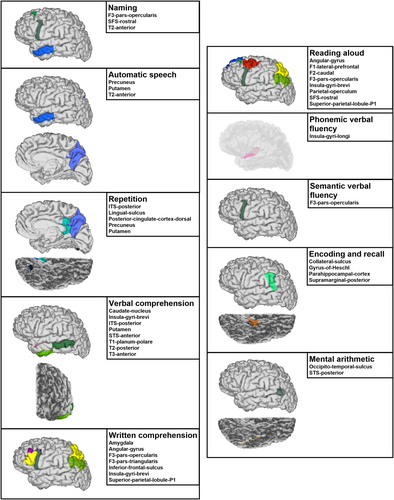
Co-occurrences between SEEG signal complexity recovery and language-domain recuperation are summarized for the 10 highest match proportions in Supplementary Table S3, with significant co-occurrences identified through permutation-based resampling illustrated in Figure 4. For naming, significant co-occurrences were observed with entropy recovery in pars opercularis, rostral superior frontal sulcus, and anterior middle temporal gyrus. Automatic speech recovery was significantly associated with complexity recovery in the anterior middle temporal gyrus, precuneus, and putamen. Repetition recovery showed significant co-occurrences with regional complexity recovery in the posterior inferior temporal and lingual sulci, posterior cingulate cortex, precuneus, and putamen. Significant verbal comprehension-entropy recuperation co-occurrences were found for the caudate nucleus, insular gyri brevi, posterior inferior and anterior superior temporal sulci, putamen, planum polare, posterior middle temporal, and anterior inferior temporal gyri. For written comprehension, significant co-occurrences were shown with complexity recovery in the amygdala, angular gyrus, pars opercularis and triangularis, inferior frontal sulcus, insular gyri brevi, and superior parietal lobule. Reading aloud-entropy recovery co-occurred significantly in the angular, superior frontal and caudal middle frontal gyri, pars opercularis, insular gyri brevi, parietal operculum, rostral superior frontal sulcus, and superior parietal lobule. Phonemic verbal fluency recovery was linked to complexity recovery in the insular gyri longi, whereas semantic verbal fluency recovery highlighted significant co-occurrences with entropy return to baseline in the pars opercularis. Encoding and recall recovery significantly co-occurred with complexity return in the posterior supramarginal gyrus, gyrus of Heschl, parahippocampal cortex, and collateral sulcus. For mental arithmetic, significant associations were identified with complexity recovery in the occipito-temporal and posterior superior temporal sulci.
We identified 9 seizures with postictal aphasia from 8 SEEG-confirmed left-hemisphere language-dominant patients where reading aloud, written comprehension, and repetition were tested, as outlined in the Materials and Methods. In all cases, ventral stream PAT was longer than dorsal stream PAT. Clinically, patients regained the ability to repeat and read aloud before written comprehension (Table 2).
| Patient ID | Repetition (R), reading aloud (RA) and written comprehension (WC) recovery order | Dorsal stream recovery time | Ventral stream recovery time |
|---|---|---|---|
| P1 | R recovered at the same time as RA, before WC | 2887.5 s | 3907.5 s |
| P2 | R recovered at the same time as RA, before WC | 32.5 s | 745 s |
| P3 | R recovered at the same time as RA, before WC | 2045 s | 2,235 s |
| P4 | R recovered at the same time as RA, before WC | 1267.5 s | 3,095 s |
| P5 | R recovered at the same time as RA, before WC | 35 s | 162.5 s |
| P5bis | R recovered at the same time as RA, before WC | 5 s | 182.5 s |
| P6 | R recovered at the same time as RA, before WC | 200 s | 1,590 s |
| P7 | R recovered at the same time as RA, before WC | 220 s | 3,900 s |
| P8 | R recovered at the same time as RA, before WC | 477.5 s | 827.5 s |
Postictal Aphasia Scale
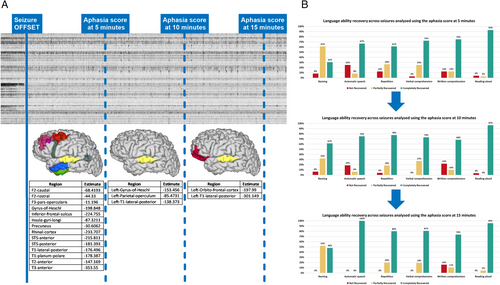
Seventy-five seizures were used to evaluate the inter-rater agreement of postictal clinical language evaluations. Substantial inter-rater agreement (Cohen κ = 0.61–0.8) was found for working memory, phonemic verbal fluency, verbal comprehension, and reading aloud tasks. For all the other language functions, inter-rater agreement was very high (Cohen κ > 0.81), the highest being for automatic speech (Cohen κ = 0.96). Furthermore, we developed the PAS, assessing verbal and written comprehension, reading aloud, naming, automatic speech, and repetition, details of which can be found in Table 3. It is based on our in-house postictal beside testing battery (see Supplementary Fig S1), partially published in its ictal form in Trebuchon et al.23 Each item is scored according to the patient's ability to perform a task: 0 (not able), 1 (partially able), and 2 (fully able). PAS scores range from 0 (no recovery, complete aphasia) to 12 (full recovery, no aphasia) and revealed Cohen's κ values of 0.97, 0.97, and 0.91 at 5, 10, and 15 minutes, respectively. A significant relationship (p < 0.05 corrected) was found between PAT of certain left-hemispheric brain regions and PAS. At 5 minutes, significant regions were widely distributed across the lateral frontal, insular, and mesial parietal cortex and the temporal lobe. The highest model estimated coefficients were observed for the anterior inferior temporal gyrus, anterior superior temporal sulcus, rhinal cortex, and inferior frontal sulcus. At 10 minutes, significant associations involved the gyrus of Heschl, parietal operculum, and the lateral-posterior part of the superior temporal gyrus. This latter region remained significant at 15 minutes, along with the orbitofrontal cortex (Fig 5A). At 5 and 10 minutes, PAS language domains indicated a global aphasia pattern, characterized by production deficits and a gradual resolution of comprehension deficits. By 15 minutes, the aphasia pattern shifted to subtle production-related impairments, notably in naming (Fig 5B).
| Criteria | Scoring | Assessment of the criteria |
|---|---|---|
| 1. Naming | 0 1 2 | The patient is able to name the shown images and/or objects. |
| 2. Automatic speech | 0 1 2 | The patient is able to automatically generate series (eg, seasons or months of the year, counting from 1 to 10) |
| 3. Repetition | 0 1 2 | The patient is able to repeat simple and complex words and phrases (eg, “Walk!”, “Catastrophe!”, “The lawyer has convinced him with his arguments!”) |
| 4. Verbal comprehension | 0 1 2 | The patient is able to follow verbal commands – both simple and complex (eg, “Raise your hands!”, “Point out with your right index first the floor, then the ceiling!”) |
| 5. Written comprehension | 0 1 2 | The patient is able to follow written commands– both simple and complex (eg, “Close your eyes!”, “After having puffed out your cheeks, stick out your tongue!”) |
| 6. Reading aloud | 0 1 2 | The patient is able to read aloud simple words, phrases and logatomes (eg, “Picture!”, “Today is sunny!”, “Snarp!”) |
- Each item is scored according to the following rule: 0 – the patient is not able to carry out the respective task. 1 – the patient is partially able out carry on the respective task. 2 – the patient is able to carry out the respective task.
Discussion
Lingering postictal deficits like aphasia may disrupt daily life more than seizures themselves, leading drug-resistant patients with infrequent events to perceive their condition as more severe.7 Better characterization of the postictal state could help address enduring challenges in epilepsy surgery – the most effective treatment for drug-resistant epilepsy – as up to 34% of patients undergoing left temporal lobe surgery experience a decline in naming and 4% in language comprehension.24 This study investigates the pathophysiology of postictal language alterations by combining clinical evaluation and signal analysis in a large cohort of patients with SEEG-explored epilepsy. To our knowledge, this is the first study to investigate in detail the electro-clinical recovery of distinct language functions during the postictal period. Additionally, we propose a novel bedside scale for assessing postictal aphasia.
Using the SEEG signal quantification method known as PAT to measure the postictal period, we demonstrated that brain complexity recovery, both globally and in the dominant hemisphere, was significantly prolonged following seizures associated with postictal aphasia compared with those without. This finding aligns with the perspective that EEG complexity is linked to cognitive performance.25 Furthermore, PAT was longer in EZN and PZN regions than in non-involved ones.
From a pathophysiological perspective, our signal complexity recovery analysis, mapped onto left-hemispheric areas and correlated with the recovery of specific language abilities, reveals distinct regional contributions to postictal language recovery, within both canonical language and broader neurocognitive systems. As highlighted by Hertrich et al,26 nearly the entire cerebral framework participates in language processing, engaging variably segregated anatomic and/or functional networks. In the following, we briefly discuss the significant regional PAT-language function recovery co-occurrences, which seem to reflect some of the basic cognitive processes supporting language abilities (detailed in Supplementary Table S4). For naming, SEEG complexity recovery was prominent in regions supporting motor-articulatory functions (frontal operculum),27 lexical-semantic decisions (middle temporal gyrus),3, 28 and word selection (superior frontal sulcus).22, 29 Co-occurrences between automatic speech and entropy recovery were found in brain areas essential for routine verbal sequences (middle temporal gyrus),30 supported by attentional focus (precuneus)31 and aided by a motor program (putamen).32 Successful postictal verbal repetition was linked to recovered complexity in regions sustaining language-attention interplay (precuneus and inferior temporal-occipital regions)31, 33 and automatic verbal output (putamen and posterior cingulate cortex).32, 34 Furthermore, verbal comprehension and complexity recovery aligned in regions involved in subcortical–cortical semantic access (caudate nucleus and putamen),35 stimulus prioritization (insula gyri brevi),28, 36 and auditory–visual cues integration for semantic decision making (anterior and posterior parts of the lobe).28, 37-40 Optimal written comprehension and reading aloud seemed to rely on recovered entropy in overlapping networks for auditory–visual cues integration at both sentence and word level (inferior frontal sulcus-gyrus),27 sensory-motor-articulatory planning and output (insular-opercular-premotor regions),27, 28, 41 adaptative monitoring and attention (superior and inferior frontal sulci, prefrontal-insular cortex),22, 29, 36, 42, 43 semantic decisions on written words (angular gyrus),28, 44 and letter-identity encoding (superior parietal lobule).45 Notably, complexity recovery in the amygdala correlated with restored written comprehension, potentially reflecting enhanced attentional salience.26 Verbal fluency was associated with entropy recovery in the insular-opercular cortex, which plays a role in articulation coordination, facilitating fluency.3, 27, 28 Moreover, auditory working memory depended on complexity recovery in regions involved in auditory perception (gyrus of Heschl), phonological rehearsal for retaining verbal information (supramarginal gyrus),28 and the basal-mesial memory temporal system.33, 46 Finally, mental arithmetic seemed to rely on entropy recovery in the posterior superior temporal and occipital-temporal sulci, structures implicated in numerical symbol recognition, and integration.47, 48
Entropy recovery analysis also supports dual-stream, cortical–subcortical, organization of language processing.3 In our cohort, the ventral stream recovered after the dorsal, possibly reflecting the greater complexity of its functions (eg, semantic processing). These findings should be interpreted with caution given the EZN organization (ipsilateral to the dominant hemisphere in all 8 patients, temporal in 7 and frontal-temporal in 1) and the limited number of seizures with clear recovery dissociation. Previously, we had shown that global brain entropy recovers more slowly in temporal lobe epilepsy than in other focal epilepsies.10 Nonetheless, such information could inform stream-specific rehabilitation protocols.49
Beyond electro-clinical recovery correlations, we developed the PAS for bedside quantification of postictal aphasia. This scale demonstrated negative fixed-effect estimates between regional recovery times and PAS scores in SEEG-confirmed left-hemisphere language-dominant patients: higher PAS scores (indicating milder deficits) were associated with faster regional signal complexity recovery. These fixed-effect estimates were substantial in absolute value (often in the hundreds) which is particularly notable given the ordinal nature and narrow range of the PAS (0–12), in contrast to the broader, continuous regional PAT scale measured in seconds (often reaching several hundred). The spatial–temporal evolution of associations between PAS scores and regional PAT values suggests that, at least in our study cohort, postictal aphasia transitions from a global impairment to more domain-specific deficits. At 5 minutes, the involvement of widely distributed regions—particularly in fronto-temporal areas—supports the presence of an early global aphasia affecting both language production and comprehension. By 10 minutes, the association with regions such as the posterior superior temporal gyrus (involved in auditory processing and comprehension),22, 28 as well as the parietal operculum (linked to integrative sensory-motor functions for speech production),27, 28 becomes more prominent. This pattern reflects a shift toward, but not necessarily limited to, a comprehension-specific dysfunction. By 15 minutes, whereas most language functions had recovered, negative associations with the orbitofrontal cortex and the posterior superior temporal gyrus suggest that the final stage of language recovery may involve higher-order integration processes, including language adaptation to social-communication context.26, 50 Furthermore, at the domain level, PAS indicated a global aphasia pattern at 5 and 10 minutes—marked by production deficits and progressively resolving comprehension deficits—that evolved into a more production-specific impairment, particularly affecting naming, by 15 minutes. Despite inter-seizure variability, these findings illustrate a fluctuating, nonlinear recovery trajectory, highlighting the dynamic interplay between regional complexity restoration and functional language recovery.
During the brief ictal period, practical and safety considerations often limit meaningful language testing.23 Additionally, altered awareness may be mistaken for language deficit, complicating interpretation. The postictal period offers a longer, more stable time-window for systematic bedside language assessment. This approach is minimally invasive, cost-effective, and yields robust electro-clinical language correlations without requiring additional modalities (eg, fMRI).6 The postictal aphasia mapping performed in the current study thus aims to establish the postictal state as a presurgical language mapping tool. Moreover, the scale we have developed, PAS, offers an easy-to-use framework to assess domain-specific postictal aphasia deficits and their severity. By identifying these deficits, PAS could guide domain-specific language rehabilitation. Recent work showed increased naming performance and left frontal activity after short-term language therapy administered during SEEG.51 PAS could also help monitor postictal deficits before and after epilepsy surgery. Prior studies have used other postictal cognitive evaluations (eg, memory) to improve presurgical planning and predict postoperative outcomes.52
Intracranial language mapping in epilepsy relies primarily on task-related direct electrical cortical stimulation and event-related high gamma activity.6 Two electrocorticography studies53, 54 used both methods to map auditory and visual naming, as well as word repetition, revealing dynamic activation patterns, age-related differences, and subnetwork organization. In another study, Ervin et al55 investigated the spatiotemporal dynamics of visual naming using high-gamma modulation and proposed a 6-phase model of language processing. They described a sequential cortical activation cascade beginning in bilateral occipital regions and transitioning to left frontotemporal areas before engaging to right frontal lobe. Moreover, resection of specific brain regions was linked to distinct postsurgical language deficits. Our approach uniquely leverages the postictal period as a naturalistic and ecologically valid perturbation. Unlike the forward, stimulus-locked designs used in earlier works, our method maps language in reverse—tracing the restitution of language abilities following seizure-induced disruption. As in the Ervin et al,55 study, we observed a multi-phase model of language processing with dynamic regional changes across a comprehensive range of language domains. However, our study focused on dominant hemisphere regions and we did not evaluate the impact of surgical resection on language functions. Furthermore, as opposed to Nakai et al,53 our mixed-effects models showed no age-related influence on brain complexity-language recovery. Nonetheless, our findings complement prior studies, and their combined use may improve presurgical mapping and prediction of postoperative outcomes. Examples associating task-related language mapping with clinical ictal-postictal elements are scarce.56
This descriptive retrospective monocentric study has several limitations. The relatively small sample size and the focus on the left hemisphere regions may limit the generalizability of these findings and may miss relevant right hemisphere information. Moreover, the study did not consider the effect of antiseizure medication type and tapering during the presurgical exploration on electro-clinical recovery. By focusing on some language tasks, our work may not capture the full spectrum of postictal aphasia (eg, jargon-aphasia) and, due to the specific seizure-related and patient-associated bedside context, not all language abilities could be tested until definite assertion of recovery. Other limitations are inherent to the SEEG method itself: patient-specific hypothesis-driven implantation, limited spatial sampling, and inter-patient variability.
This study offers novel insights into the pathophysiology of postictal aphasia by combining SEEG-based signal complexity analysis with bedside language testing. The postictal state holds significant untapped potential for presurgical language mapping and the development of anatomically and functionally informed rehabilitation protocols.
Acknowledgments
The authors thank the anonymous reviewers for their insightful comments, which helped improve the scientific quality of this work. We are especially grateful to Véronique Sabadell for coordinating the speech therapy team and Estelle Sangouard for her work on the development of the language testing battery. We thank nurse practitioners Mireille Fazzolari, Muriel Neumayer, and Catherine Giampietri for their crucial involvement in the postictal management and bedside testing of the included patients. We acknowledge the clinical teams involved in patient care: Dr Julia Makhalova, Dr Stanislas Lagarde, Dr Francesca Pizzo, Dr Francesca Bonini, Dr Aileen McGonigal, Dr Lisa Vaugier, Dr Sandrine Aubert, Dr Nathalie Villeneuve, Dr Anne Lepine, and their teams. We also thank Professor Didier Scavarda, Dr Romain Carron, and their teams for the surgical management of included patients. We are grateful to Bernard Giusiano and Aurore Semeux-Bernier for their guidance on the statistical analyses. Finally, we acknowledge the NeuroMarseille Graduate School for overseeing the doctoral training of author Ionuț-Flavius Bratu. This work, carried out within the Institut de Neurosciences des Systèmes and the Institute of Convergence ILCB, has benefited from support from the French government under the “France 2030” investment plan managed by the French National Research Agency (Reference: ANR-16-CONV000X/ANR-17-EURE-0029 and ANR-16-CONV-0002), from Excellence Initiative of Aix-Marseille University – A*MIDEX (Reference: AMX-19-IET-004) and from the VESTISELF project (Reference: ANR-19-CE37-0027).
Author Contributions
I.F.B., F.B., and A.T. contributed to conception and design of the study. All authors contributed to the acquisition and analysis of data and to drafting the text or preparing the figures.
Potential Conflicts of Interest
The authors report no potential conflict of interest related to this work.
Open Research
Data Availability
GARDEL, AnyWave software and dedicated plug-ins (cEI) are available at https://meg.univ-amu.fr. The VEP atlas is available at https://ins-amu.fr/vep-atlas. Data that support our findings are available from the corresponding author upon reasonable request. <zbmrule>




