Pediatric RNS Lead Migration: Wandering Eyes or Electrode?
Neuromodulation with responsive neurostimulation (RNS, NeuroPace, Mountain View, CA) is increasingly being used off-label in pediatric drug-resistant epilepsy (pDRE).
A 7-year-old boy, with RNS depth electrodes targeting bilateral thalamic centromedian nucleus (CM); developed paroxysmal bilateral esotropia (left > right) 52 weeks after initial implantation. Esotropia worsened over 3 weeks and was discovered to be caused by a ventrally migrated left CM electrode.
Bilateral CM implantation of narrowly spaced (3.5 mm) 13.6 mm RNS electrodes was achieved using direct magnetic resonance imaging (MRI) visualization and Robotic Surgical Assistant (ROSA) guidance after extensive presurgical evaluation. The RNS battery was housed over the right parietal region, with the left CM electrode inserted via a 2.5 mm left frontal burr hole. A lead protector sheath covered the electrode and was secured with a “dogbone” titanium cranial plate provided by NeuroPace. A 50% seizure reduction was achieved after 6 months, and stepwise charge density increases were well tolerated without adverse effects. At 40 weeks, the mother reported increased seizures and at 50 weeks, “tilting head to one side” and drifting of the left eye; which seemed to be “floating around.” This was initially attributed to a refractive error. At 52 weeks, the family reported an episode of eyes “going in all directions” and occasional loss of vision. Urgent head computed tomography (CT) was initially reported as unchanged. The patient displayed left head tilt and intermittent right head turn. Neurologic examination, notable for intermittent esotropia (left > right; see Fig 1A) with normal mental status, pupillary response, and full eye excursions directed localization hypothesis to the midbrain. CT images were re-examined with co-registration to preoperative MRI and ROSA coordinates, confirming an 8 mm ventral and medial migration of the left CM electrode into the midbrain (see Fig 1A).
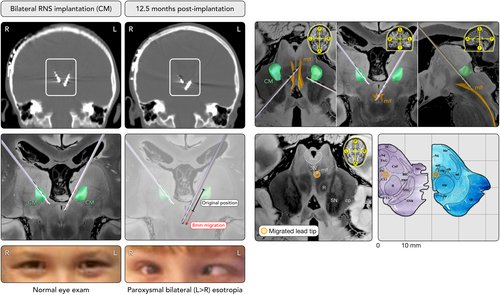
Comparison to anatomic atlases using Lead-DBS software,1 including previously published atlases of brainstem tractography1, 2 and histology3 (see Fig 1B), revealed that the left CM tip had crossed the midline and was located within the medial longitudinal fasciculus (MLF), immediately adjacent to the oculomotor nucleus and approximately 3 mm anterior to the periaqueductal gray area.
Within a few minutes of disabling RNS stimulation, the eye movements normalized. During surgical admission for left CM electrode repositioning, the abnormal eye movements were reproduced (medial deviation L > R, non-reproducible downward deviation without pupil changes) with stimulation of the 3 deepest left CM electrode contacts, but not with the remaining 4 right CM or the most superficial left CM contact. Intraoperative exposure revealed distal migration of the left CM electrode sheath protector from under the securing “dogbone.” The dogbone and screw thus failed to adequately secure the electrode, allowing deeper migration.
Discussion
Depth lead migration in pDRE has not been described. Lead migration is the second most common delayed hardware complication in adult DBS, with an overall prevalence of < 2.0%. Most migrations are dorsally directed and discovered when DBS efficacy is lost.4 Although many neurosurgeons use the Navigus Stimloc Burr Hole Cover system (Medtronic, Minneapolis, MN) for electrode fixation, most pediatric neurosurgeons implanting RNS use the “dogbone” technique with the protective sheath. The “Stimloc” requires a larger burr hole whereas the “dogbone” technique utilizes a smaller stereoelectroencephalography (SEEG) burr hole (preferable in pediatric neurosurgery).
The exact timing or trigger for migration in our patient remains unclear. Because RNS titrations are traditionally performed every 10 to 12 weeks, we were still in the active phase of therapy optimization, and lead migration as a possibility for increased seizure frequency was never considered.
With individual centers still operating in silos, urgent and widespread dissemination of late complications like ours is crucial. The exponential increase of intracranial neuromodulation in pediatrics requires vigilance for clinical changes, particularly those that may be mistaken for disease progression rather than unintended complications of treatment.
Acknowledgments
The authors would like to extend their gratitude to the family members for supplying photographs and agreeing to participate in this project.
Author Contributions
C.J. contributed to the conception and design. C.J., D.V., A.V.P., A.W., C.D., D.S., A.T., and R.S. contributed to acquisition and analysis of data. C.J. and A.W. contributed to drafting the text and preparing figures.
Potential Conflicts of Interest
None of the authors have anything to report.
Open Research
Data Availability
Related data can be made available to other investigators upon request to the corresponding author.




