Cognitive Performance in Early Neuronal Synuclein Disease with Hyposmia but without Motor Disability: Association with Dopamine Deficiency and Isolated Rapid Eye Movement Sleep Behavior Disorder
Members of the Parkinson's Progression Markers Initiative are available as an on online supplementary file, Data S1.
Abstract
Objective
To determine the impact of dopamine deficiency and isolated rapid eye movement (REM) sleep behavior disorder (iRBD) on cognitive performance in early neuronal α-synuclein disease (NSD) with hyposmia but without motor disability.
Methods
Using Parkinson's Progression Markers Initiative baseline data, cognitive performance was assessed with a cognitive summary score (CSS) derived from robust healthy control (HC) norms. Performance was examined for participants with hyposmia in early NSD-Integrated Staging System (NSD-ISS), either stage 2A (cerebrospinal fluid α-synuclein seed amplification assay [SAA]+, dopamine transporter scan [DaTscan]−) or 2B (SAA+, DaTscan+).
Results
Participants were stage 2A (n = 101), stage 2B (N = 227), and HCs (n = 158). Although stage 2 had intact Montreal Cognitive Assessment scores (mean [SD] = 27.0 [2.3]), stage 2A had a numerically worse CSS (z-score mean difference = 0.05, p = NS; effect size = 0.09) and stage 2B a statistically worse CSS (z-score mean difference = 0.23, p < 0.05; effect size = 0.40) compared with HCs. In stage 2A, hyposmia alone was associated with normal cognition, but those with comorbid iRBD had significantly worse cognition (z-score mean difference = 0.33, p < 0.05, effect size =0.50). In stage 2B, hyposmia alone had abnormal cognition (z-score mean difference = 0.18, p = 0.0078, effect size = 0.29), and superimposed iRBD had a statistically significant additive effect.
Interpretation
Using a novel CSS, we demonstrated that hyposmia is associated with cognitive deficits in prodromal NSD without motor disability, particularly when comorbid dopamine system impairment or comorbid iRBD is present. Therefore, it is critical to include and assess cognition at all stages when studying synuclein disease, even in the absence of motor disability. ANN NEUROL 2025
Significant cognitive impairment is a common concern in Parkinson's disease (PD), with up to 80% of patients developing dementia in the long-term.1, 2 Mild cognitive impairment is also frequent,3 even in early diagnosed PD.4-6 Subtle cognitive changes can also appear in prodromal states, such as hyposmia7 or isolated rapid eye movement (REM) sleep behavior disorder (iRBD),8 the latter being linked to both PD and dementia with Lewy bodies (DLB). One study observed global and specific cognitive deficits in persons with both hyposmia and iRBD.9 We were interested in assessing cognitive deficits in individuals before they developed motor disability.
Recently, we proposed a research biological definition and staging system for neuronal α-synuclein disease (NSD-ISS),10 with NSD defined by the presence of biomarker of pathological neuronal α-synuclein species as the primary biological anchor, and dopaminergic neuronal dysfunction as a subsequent additional biological anchor. NSD also incorporates an integrated staging system (ISS), rooted in the aforementioned biological anchors plus severity of functional impairment caused by clinical signs or symptoms along 3 tracks (ie, motor, non-motor, and cognitive). The presence of subtle clinical signs marks the transition to NSD-ISS stage 2 and beyond, with stage 2 characterized by subtle signs or symptoms (ie, hyposmia or iRBD, or early motor [subthreshold parkinsonism or on PD meds] or cognitive symptoms [Movement Disorders Society Modified Unified Parkinson's Disease Rating Scale (MDS-UPDRS) part 1.1 score = 1 and Montreal Cognitive Assessment (MoCA) score ≥ 25]), but without functional impairment, particularly from motor impairment (ie, prodromal disease).10, 11 Stage 2A features α-synuclein positivity without dopaminergic neuronal dysfunction (negative), whereas stage 2B is α-synuclein positive with dopaminergic dysfunction present (positive). In Parkinson's Progression Markers Initiative (PPMI) the presence of neuronal α-synuclein is currently determined by a positive cerebrospinal fluid (CSF) α-synuclein seed amplification assay (SAA), and dopaminergic neuronal dysfunction by a single-photon emission computerized tomography (SPECT) dopamine transporter (DAT) scan (DaTscan).
When assessing cognitive abilities, an option that combines the richness of a neuropsychological battery with the simplicity of a single test score is a cognitive summary score (CSS). In the Parkinson Associated Risk Syndrome (PARS) study, a CSS captured subtle cognitive changes in prodromal PD (ie, hyposmia and positive DaTscan).7, 12 Using the original PPMI cognitive battery (5 detailed, well-established cognitive tests),13 we recently implemented a robust norming process using baseline PPMI data to enhance sensitivity in detecting cognitive changes in de novo PD compared with healthy controls (HCs), similar to updates made for the Dementia Rating Scale.14
These analyses aimed to evaluate cognition in early-stage NSD (ie, stage 2 without motor disability) using the proposed NSD-ISS criteria. Participants had (1) a positive CSF neuronal α-synuclein SAA test, with and without dopaminergic neuronal dysfunction, and (2) hyposmia. We hypothesized that early-stage NSD and hyposmia would show worse cognitive performance compared with HCs, and that both co-morbid iRBD and dopaminergic neuronal dysfunction would increase the severity of cognitive deficits. Although impaired cognition has been reported in prodromal PD, we are not aware of any studies that have examined cognitive abilities in patients with early-stage synuclein disease without motor disability, but confirmed by the presence of a positive α -synuclein SAA test, using a novel CSS, or examining the impact of hyposmia, iRBD, and dopaminergic system deficit all in the same cohort. Confirming our hypothesis would validate the current inclusion of a cognitive track in NSD-ISS, from the earliest disease stages, and more importantly highlight the importance of including cognition as an outcome measure in disease-targeting randomized controlled trials for early stage NSD, even in the absence of motor disability.
Methods
We adhered to the STROBE checklist for cross-sectional studies.15
Enrollment Cohorts
The PPMI study and enrollment cohorts, all of whom are represented in these analyses, have been extensively described.16, 17 The study is in compliance with all relevant ethical regulations. Informed consent was obtained from all human participants. The current PPMI Clinical study protocol (002) received initial WCG approval (institutional review board [IRB] tracking 20200597) on April 20, 2020. The previous PPMI Clinical protocol (001) received initial IRB approval on May 7, 2010, by the University of Rochester Research Subjects Review Board (RSRB) (00031629) and was closed by the RSRB on March 9, 2021.
Individuals without a known diagnosis of PD are enrolled in the study based on risk factors for or signs/symptoms of potential early synucleinopathy (also called prodromal disease), including hyposmia, iRBD, and relevant genetic variants carriers as described previously.11 Inclusion criteria for de novo PD participants included: (1) an asymmetric resting tremor or asymmetric bradykinesia, or at least 2 symptoms out of resting tremor, bradykinesia, and rigidity; (2) a recent clinical diagnosis of PD (mean [standard deviation, SD] duration from diagnosis = 8.5 [7.3] months); (3) being untreated with PD medications at study entry; (4) evidence of dopaminergic neuronal dysfunction based on DaTscan imaging; and (5) being non-demented by site investigator clinical determination. Participants with a DaTscan without evidence for dopaminergic deficit (SWEDDs) have also been previously described.17
HCs at enrollment were required to (1) not have clinically significant neurological dysfunction, (2) not have a first-degree relative with PD, and (3) have a MoCA18 score ≥27. For the purposes of these analyses, HCs could not have hyposmia and had to have a negative CSF neuronal α-synuclein SAA test. Finally, to generate the internally derived norms for the CSS, we created a robust HC subgroup that was also required to have a year 1 MoCA score of ≥26 and not have had more than a 2-point drop in their MoCA score between baseline and year 1. This group is hereafter called the HC subgroup.
Redefining PD Participants Using NSD-ISS
All the aforementioned enrollment cohorts were reclassified to their NSD-ISS stage. CSF was collected as previously described and assayed for neuronal α-synuclein with SAA test as described.19 Dopaminergic dysfunction was assessed with DaTscan as described.17
Only participants classified as stage 2 (ie, those with subtle signs or symptoms [ie, no functional impairment], also called prodromal disease) were included in these analyses. Therefore, participants classified as Stage 3 (slight signs or symptoms [ie, slight functional impairment])10, 11 and above were excluded from these analyses.
Participants with normal and abnormal DaTscan results were classified as stages 2A and 2B, respectively. For the purposes of these analyses and consistent with recent NSD analyses,11 a DaTscan deficit was defined as <75% age-and sex-expected lowest putamen specific binding ratio. In addition, the following inclusion criteria were also applied: participants were required to: (1) have hyposmia, defined by an age/sex-adjusted University of Pennsylvania Smell Identification Test (UPSIT) ≤15th percentile (those who were normosmic or had a missing UPSIT were excluded); (2) not be on medication for treating the symptoms of PD; (3) have completed all 5 tests in the original PPMI cognitive battery; and (4) not have cognitive, motor, or other non-motor features qualifying for Stage 3 or higher. Last, participants were classified into “hyposmia only” and comorbid “hyposmia and iRBD” subgroups. To make these subgroups as “pure” as possible, only participants without any evidence of possible RBD (defined by a score ≥6 on the RBD screening questionnaire, self-reporting an RBD diagnosis without polysomnogram (PSG) confirmation, or self-reporting dream enactment behavior) were included in the hyposmia group, and only participants with PSG-confirmed RBD were included in the iRBD group. NSD participants with a normal DaTscan were stage 2A, and those with an abnormal DaTscan were stage 2B.
Mapping the biologically defined NSD-ISS stages to the 3 primary PPMI enrollment cohorts (ie, de novo PD, prodromal PD, and HCs), all HCs were enrolled as HCs, approximately half of stage 2A participants were enrolled as hyposmics and most of the rest qualified on the basis of having iRBD (and also were subsequently determined to have comorbid hyposmia), and approximately half of the Stage 2B participants were enrolled as sporadic PD, with nearly all the rest enrolled as hyposmic or having iRBD.
CSS
Baseline data were used, at which point those participants enrolled in the PD cohort were recently diagnosed and had not yet begun treatment with PD medication. The following steps were taken: (1) creating a robust HC subgroup that did not demonstrate cognitive decline over time; (2) using the HC subgroup to create regression-based internally derived standardized scores (z-scores) for 6 cognitive scores across 5 tests, with the standardized scores controlling age, sex, and education; and (3) creating a CSS by averaging all standardized test z-scores. The original cognitive battery was used to create the CSS to optimize the amount of data available for analyses. This original battery was composed of the Hopkins Verbal Learning Test-Revised (immediate and delayed free recall scores),20 the Benton Judgment of Line Orientation-15 item version,21 Symbol-Digit Modalities Test,22 Letter-Number Sequencing,23 and semantic (animal) fluency.24 Together these tests assess memory, visuospatial function, information processing speed, executive function, working memory, and language. Global cognition was assessed with the MoCA.18
Analyses
Statistical analyses were performed using SAS v9.4 (SAS Institute, Cary, NC; sas.com; RRID:SCR_008567). Using PPMI baseline data participant characteristics were compared between the stage 2A, stage 2B, and HC groups using mean (SD) and median (interquartile range) for continuous measures and frequency (percentage) for categorical measures. A t test was performed for continuous measures and a χ2 or Fisher's exact test was performed for categorical measures to compare characteristics across the 3 groups. Next, a t test was used to assess p-values for difference in mean MoCA score between stage 2A and stage 2B groups. Cohen's d effect sizes were also evaluated and reported.
To evaluate the impact of superimposed iRBD (in addition to hyposmia) on cognitive performance in stage 2 participants, 2-sample t tests were performed to compare the CSS between hyposmia only versus comorbid hyposmia and iRBD participants. These subgroups were also compared to the HC subgroup. The associated Cohen's d effect sizes were also evaluated and reported. This analysis was performed for the overall stage 2 group, as well as based on their dopaminergic dysfunction status (ie, separately within stage 2A and stage 2B subgroups).
Results
Cohorts
The cohort comprised of NSD stage 2 participants (2A n = 101, 2B n = 227) and HCs (n = 158). Of the 328 participants with stage 2 NSD and hyposmia, 27.7% (n = 91) had comorbid iRBD, representing 43% of the stage 2A cohort and 21% of the stage 2B cohort.
Demographic and Clinical Characteristics
Regarding characteristics, 60 to 65% of all 3 cohorts were male, consistent with PD research cohorts, and all were predominately white (91–96%) and non-Hispanic (94–99%) (Table 1). HCs were significantly younger, but had a wider age range, than stage 2 participants. All stage 2 participants met non-motor criteria for stage 2, as an inclusion criterion for these analyses was presence of hyposmia. Participants can also meet stage 2 criteria on the basis of subtle cognitive changes (ie, MDS-UPDRS part 1 cognition item = 1 and MoCA score ≥25), and 17% of stage 2A and 16% of stage 2B participants met cognition criteria for stage 2, compared with 9% of HCs. Close to 60% of stage 2B participants met the motor criteria for stage 2. 38.5% (35/91) of participants with co-morbid iRBD were taking clonazepam as symptomatic therapy, compared with <1% of hyposmia alone participants (1/237).
| Variable | Stage at baseline | p-values | ||||
|---|---|---|---|---|---|---|
| HCa (n = 158) | 2A (n = 101) | 2B (n = 227) | HC vs 2A | HC vs 2B | 2A vs 2B | |
| Sex (M), n (%) | 96 (61) | 63 (62) | 140 (62) | 0.7944 | 0.8562 | 0.9038 |
| Race (White), n (%) | 143 (91)b | 93 (94)b | 218 (96)b | 0.4797 | 0.0421d | 0.3734 |
| Ethnicity (non-Hispanic), n (%) | 156 (99) | 94 (95)b | 208 (94)b | 0.1112 | 0.0159d | 0.6597 |
| Age at baseline | ||||||
| Mean yr (SD) | 58.96 (11.69) | 66.93 (5.86) | 64.98 (8.23) | <0.0001d | <0.0001d | 0.0156d |
| Median yr (IQR) | 60.37 (52.98–67.04) | 66.86 (62.51–70.49) | 65.52 (60.62–70.67) | |||
| Education | ||||||
| Mean yr (SD) | 16.1 (3.0) | 16.9 (3.3) | 16.4 (3.4)b | 0.0324d | 0.3802 | 0.1677 |
| GDS-15 score | ||||||
| Mean (SD) | 1.2 (2.2) | 1.4 (2.1) | 1.3 (2.0) | 0.6346 | 0.6377 | 0.9073 |
| MDS-UPDRS part 1 apathy score, n (%) | ||||||
| Score ≥1 | 7 (4)b | 8 (8) | 26 (11) | 0.2461 | 0.0162d | 0.3325 |
| Meets cognitive criteria for stage 2, n (%) | ||||||
| Item 1.1 = 1 but MoCA ≥25 | 14 (9)c | 17 (17) | 37 (16) | 0.0564 | 0.0461d | 0.9045 |
| Meets motor criteria for stage 2, n (%) | ||||||
| Subthreshold parkinsonism OR PD meds | 5 (3) | 9 (9) | 136 (60) | 0.0461d | <0.0001d | <0.0001d |
| Meets non-motor criteria for stage 2, n (%) | ||||||
| Hyposmia or iRBD | 18 (11) | 101 (100) | 227 (100) | N/A | N/A | N/A |
| Non-motor subgroup, n (%) | ||||||
| Hyposmia and iRBD | 0 (0) | 43 (43) | 48 (21) | N/A | N/A | <0.0001d |
| Hyposmia only | 18 (100)c | 58 (57) | 179 (79) | |||
| Cohort/subgroup at enrollment, n (%) | ||||||
| HC | 158 (100) | 9 (9) | 1 (<1) | N/A | N/A | <0.0001d |
| Sporadic PD | 0 (0) | 0 (0) | 116 (51) | |||
| Genetic PD | 0 (0) | 0 (0) | 1 (<1) | |||
| iRBD | 0 (0) | 43 (43) | 48 (21) | |||
| Hyposmia | 0 (0) | 44 (44) | 58 (26) | |||
| Genetic NMC | 0 (0) | 5 (5) | 2 (1) | |||
| SWEDD | 0 (0) | 0 (0) | 1 (<1) | |||
- a Robust HC criteria: (1) not S+ (2) MoCA ≥27 at BL (3) MoCA ≥26 at 1Y and (4) no more than a 2-point drop in MoCA between BL and 1Y.
- b Two stage 2A participants, 1 stage 2B participant and 1 HC participant had missing race information. Two stage 2A participants and 5 stage 2B participants had missing ethnicity information. Three stage 2B participants had missing education information and 1 HC participant had a missing MDS-UPDRS part 1 apathy score.
- c There was 1 HC participant who was not evaluable for cognitive and non-motor criteria because of missing data; there was 1 HC participant who met cognitive criteria for Stage 3 that is, Item 1.1 = 2 and MoCA ≥25.
- d p-Value from tests for differences across the three groups. χ2 or Fisher's exact tests were used for categorical variables. T tests were used for continuous variables and Pooled/Satterthwaite p-values were reported depending on if equal variances assumption is met/not met. Statistically significant at p < 0.05. The level of significance is determined based on the assumption that having sample sizes >100 in each group and performing three comparison tests is sufficient to control for Type I error.
- GDS-15 = 15-item Geriatric Depression Scale; HC = healthy controls; IQR = interquartile range; IRBD = isolated rapid eye movement (REM) sleep behavior disorder; M = male; MDS-UPDRS = Movement Disorders Society Modified Unified Parkinson's Disease Rating Scale; meds = medication; MoCA = Montreal Cognitive Assessment; NMC = non-manifesting carriers; NSD = neuronal α-synuclein disease; PD = Parkinson's disease; SD = standard deviation; SWEDD = participants with a DaTscan without evidence for dopaminergic deficit.
Cognitive Performance in Stage 2 NSD with Hyposmia
For cognition, MoCA scores were slightly lower for stage 2A and 2B compared with the HC subgroup, which is not surprising given that MoCA score <27 was an exclusion criterion for the HC subgroup (Table 2). However, the mean MoCA scores for all 3 groups exceeded the cut-off score (score ≥26), although the SD of scores for stages 2A and 2B were almost twice that of the HC group.
| Variable | Subgroup | p-values | ||||
|---|---|---|---|---|---|---|
| HCa (n= 158) | Stage 2A (n = 101) | Stage 2B (n = 227) | Stage 2A vs HC | Stage 2B vs HC | Stage 2B vs 2A | |
| MoCA, mean (SD) | 28.3 (1.1) | 26.9 (2.1)c | 27.1 (2.4) | N/A | N/A | 0.2321 |
| Median (IQR) | 28 (27–29) | 27 (25–28) | 28 (26–29) | |||
| Cognitive summary score, mean (SD) | −0.02 (0.61) | −0.07 (0.68)c | −0.25 (0.65)c | 0.4917 | 0.0003b | 0.0214b |
| Median (IQR) | −0.03 (−0.46 to 0.40) | −0.10 (−0.57 to 0.41) | −0.25 (−0.68 to 0.24) | |||
- a Robust HC criteria: (1) not S+ (2) MoCA ≥27 at BL (3) MoCA ≥26 at 1Y and (4) no more than a 2-point drop in MoCA between BL and 1Y.
- b Statistically significant at p < 0.05.
- c MoCA score was missing for 2 stage 2B participants; Cognitive summary score was missing for 1 HC participant and not evaluable for 3 stage 2B participants because of missing education information (required for standardization of test z-scores).
- HC= healthy controls; IQR = interquartile range; MoCA = Montreal Cognitive Assessment; SD = standard deviation.
For the CSS, stage 2A scored numerically worse (z-score mean difference = 0.05, p = NS; effect size = 0.09) and stage 2B scored statistically worse (z-score mean difference = 0.23, p < 0.05; effect size = 0.38) than the HC subgroup. Stage 2B scored statistically worse than stage 2A (z-score mean difference = 0.18, p < 0.05).
Cognitive Performance in Stage 2 NSD with Hyposmia by Comorbid iRBD
Considering all of stage 2 NSD as a single group, participants with hyposmia only (ie, no iRBD) had non-statistically significant worse cognitive performance than the HC subgroup (z-score mean difference = 0.12, p = NS, effect size = 0.18), and having superimposed iRBD nearly doubled the magnitude of cognitive deficits (z-score mean difference for hyposmia + iRBD vs hyposmia alone = 0.24, p < 0.05, effect size = 0.36) (Table 3).
Examining NSD stage 2A participants (ie, without dopaminergic neuronal dysfunction), those with hyposmia only had normal cognition, but the addition of comorbid iRBD led to a statistically significant increase in cognitive deficit (z-score mean difference for hyposmia + iRBD vs hyposmia alone = 0.33, p < 0.05, effect size = 0.50) (Table 3; Fig 1).
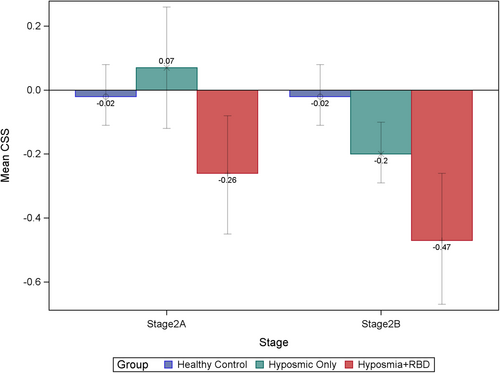
In NSD stage 2B participants (ie, with dopaminergic neuronal dysfunction), those with hyposmia alone now demonstrated significant cognitive deficits (z-score mean difference = 0.18, p < 0.05, effect size = 0.29), and superimposed iRBD had an additional statistically significant effect (z-score mean difference = 0.27, p < 0.05, effect size =0.41) (Table 3; Fig 1).
| Overall stage 2 | p-values | |||||
|---|---|---|---|---|---|---|
| Variable | HC (n = 158) | Hyposmia only (n = 237) | Hyposmia + iRBD (n = 91) | Hyposmia only vs HC | Hyposmia + iRBD vs HC | Hyposmia + iRBD vs hyposmia only |
| Cognitive summary score | 0.0797 | <0.0001b | 0.0036b | |||
| Mean (SD) | −0.02 (0.61) | −0.13 (0.66)a | −0.37 (0.67) | |||
| Median (IQR) | −0.03 (−0.46, 0.40) |
−0.12 (−0.63, 0.34) |
−0.38 (−0.79, 0.05) |
|||
| Stage 2A | ||||||
|---|---|---|---|---|---|---|
| Hyposmia only (n = 58) | Hyposmia + iRBD (n = 43) | |||||
| Cognitive summary score | 0.3838 | 0.0193b | 0.0139b | |||
| Mean (SD) | −0.02 (0.61) | 0.07 (0.71) | −0.26 (0.60) | |||
| Median (IQR) | −0.03 (−0.46, 0.40) |
0.15 (−0.54, 0.53) |
−0.33 (−0.65, 0.03) |
|||
| Stage 2B | ||||||
|---|---|---|---|---|---|---|
| Hyposmia only (n = 179) | Hyposmia + iRBD (n = 48) | |||||
| Cognitive summary score | 0.0078b | <0.0001b | 0.0116b | |||
| Mean (SD) | −0.02 (0.61) | −0.20 (0.63)a | −0.47 (0.72) | |||
| Median (IQR) | −0.03 (−0.46, 0.40) |
−0.21 (−0.64, 0.26) |
−0.46 (−0.86, 0.06) |
|||
- a There were 3 stage 2B participants in the hyposmia only group who had missing cognitive summary scores.
- b Statistically significant at p < 0.05.
- HC = healthy controls; IQR = interquartile range; IRBD = isolated rapid eye movement (REM) sleep behavior disorder; NSD = neuronal α-synuclein disease; SD = standard deviation.
Discussion
Applying a novel CSS, we explored the impact of hyposmia, isolated REM sleep behavior disorder, and dopaminergic system deficits on cognitive performance within the same early-stage, NSD cohort without significant motor disability. Our research addresses a significant gap in the literature and holds important implications for clinical management and clinical trial design in prodromal disease. Classifying hyposmics with prodromal or de novo PD as early-stage NSD, we found that presence of NSD alone, without concomitant dopaminergic dysfunction, is not associated with cognitive deficits, unless comorbid iRBD is present. Moreover, once dopaminergic dysfunction is present, hyposmia in and of itself is associated with cognitive changes and superimposed iRBD continues to have an additive effect. The findings suggest that presence of abnormal synuclein biology in hyposmia does not appear to be associated with cognitive changes until other features emerge, such as dopamine deficiency and iRBD.
Previous research has found that persons in the general population with hyposmia plus dopaminergic system dysfunction (ie, DaTscan deficit) have detectable cognitive deficits compared with persons without these 2 characteristics.7, 12 Our results extend these findings to persons with diagnosed early-stage NSD, classified on the basis of having a positive α-synuclein SAA test, but with only subtle clinical symptoms and no functional impairment. Interestingly, without dopaminergic neuronal dysfunction cognitive performance was normal in persons with NSD and hyposmia only, suggesting that synucleinopathy at this stage may not be widespread or targeted enough to lead to changes in cognition. Under this hypothesis, cognitive deficits in hyposmics emerge only after dopaminergic dysfunction also occurs, which could reflect either that more widespread brain synuclein disease has developed in parallel or that the dopamine system has a direct role in cognitive decline in NSD, as previously demonstrated in PD using PPMI data.25 This is also consistent with the brain-first versus body-first disease model of PD,26 in which brain-first disease (eg, hyposmia alone) have normal cognition in early disease.
Numerous studies have reported cognitive deficits in patients with iRBD,27 and that cognitive deficits in this disorder predict conversion to dementia with Lewy bodies (DLB) rather than PD.28, 29 We now demonstrate that the presence of comorbid iRBD in early-stage NSD with hyposmia is associated with clear cognitive changes. Additionally, in contrast to the findings in hyposmia alone, iRBD participants did not require that a dopaminergic deficit be present to demonstrate cognitive decline, suggesting either that iRBD is associated with more diffuse synuclein disease even in early disease, co-occurs with significant non-dopaminergic neurotransmitter deficits that contribute to cognitive impairment, or commonly co-occurs with other disease pathology impacting cognition (eg, Alzheimer's disease (AD), which is known to be common in Lewy body dementias30). Although dopaminergic deficits in iRBD predict faster conversion to α-synucleinopathy,31 they do not appear essential to the cognitive decline that occurs in this population. This finding is also consistent with the brain-first versus body-first PD model,26 in which body-first disease (eg, presence of iRBD) is associated with early cognitive decline.
As alluded to above, the neurobiological underpinnings of cognitive changes in prodromal Lewy body disorders, called here early-stage NSD, appear complex. Overall, just the presence of synuclein disease and prodromal clinical features, in this case hyposmia and to some extent comorbid iRBD, is associated with detectable cognitive differences, suggesting a possible role for synuclein in cognitive performance, even in early disease. The addition of dopaminergic deficiency has an additive effect on cognitive impairment, already reported in early PD for fronto-striatal executive impairments.32 Additionally, the association with cognitive dysfunction appears more profound for iRBD compared with hyposmia alone, suggesting that the cognitive changes seen in these 2 disorders may have different neurobiological underpinnings. This is consistent with iRBD having a more malignant cognitive course than hyposmia, with more frequent conversion to DLB in iRBD33 than is seen with hyposmia, which progresses primarily to PD.34, 35 As mentioned previously, these differences are also included the brain-first versus body-first model of Lewy body disorders,36 with the former including hyposmia as an initial symptom and only limited cognitive decline early on, and the latter including iRBD as an initial disorder with significant early cognitive decline.26
Strengths of the study include a focus on 2 prodromal conditions for Lewy body disorders (ie, hyposmia and iRBD), a large sample size, inclusion of both biological anchors for NSD-ISS (ie, CSF α-synuclein SAA and DaTscan), and use of a CSS derived from a detailed cognitive battery, normed using an internal, robust HC group, and controlled for effects of age, sex, and education. Regarding the last point, it is interesting to note that although the MoCA score was lower and the percentage meeting cognitive criteria for stage 2 was higher in stage 2A than in stage 2B, the CSS score was lower in stage 2B, suggesting a benefit in using a more sensitive cognitive measure. Limitations include the lack of racial and ethnic diversity in PPMI, which limits generalizability. Other limitations include not having an adequate sample size of iRBD participants without hyposmia as a comparator group, allowing stage 2 NSD to have been enrolled in the PPMI study as part of different clinical cohorts, and only having a dichotomous outcome for SAA status. Regarding the latter point, there is preliminary evidence that a continuous measure of neuronal α-synuclein SAA (ie, kinetic parameters) is associated with cognitive performance in PD,37 therefore, future research can examine if there is a correlation between this measure and cognitive performance in stage 2 NSD. Additionally, presence of comorbid AD pathology and other reported biological contributors to cognitive decline in PD dementia or DLB can be examined to see if they help explain the greater cognitive deficits seen in participants with comorbid iRBD. For instance, we were only able to assess dopaminergic dysfunction in these analyses, but there is emerging literature about the occurrence of non-motor subtypes in PD,38 including cholinergic and noradrenergic subtypes that are associated with cognitive deficits.39, 40 Future research can assess if other clinical or biological features of proposed PD subtypes are associated with cognitive changes in early NSD.
The discovery of detectable cognitive differences in early-stage NSD with hyposmia, but without motor disability, particularly in those with dopaminergic deficiency or comorbid iRBD, underscores the necessity of including in NSD-ISS, at all disease stages, a cognitive track for symptom progression and functional impairment. The results highlight the importance of carefully assessing cognition, with more than a screening instrument, in longitudinal observational studies and in randomized clinical trials of prodromal synuclein disease.
Acknowledgments
Data used in the preparation of this article were obtained from the PPMI database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org. PPMI- a public-private partnership- is funded by The Michael J. Fox Foundation for Parkinson's Research and funding partners. See Data S1 for full list of PPMI Collaborators.
Author Contributions
D.W., M.C.B., M.K.Y., R.D., K.M., C.T., T.S., A.S., D.G., L.M.C., C.C., K.M., K.L.P., T.F., B.M., E.G.B., K.K., M.F., S.C., R.N.A., and A.V. contributed to conception and design of the study; D.W., A.R.N., R.K., C.K., M.C.B., M.K.Y., R.D., K.M., C.T., T.S., A.S., D.G., L.M.C., C.C., K.M., K.L.P., T.F., B.M., E.G.B., K.K., M.F., S.C., R.N.A., and A.V. contributed to acquisition and analysis of data; D.W., A.R.N., R.K., M.C.B., M.K.Y., R.D., T.S., A.S., L.M.C., K.M., K.L.P., E.G.B., K.K., and R.N.A. contributed to drafting the manuscript or preparing the figures. See Data S1 for full list of PPMI collaborators.
Potential Conflicts of Interest
A.S. declares consultancy with GE Healthcare. K.P. was on a scientific advisory board for Amprion.
Open Research
Data Availability
Data used in the preparation of this article were obtained on February 5, 2024, from the PPMI database (www.ppmi-info.org/access-data-specimens/download-data), RRID:SCR_006431. For up-to-date information on the study, visit www.ppmi-info.org. The analyses were conducted by the PPMI Statistics Core and used actual dates of activity for participants, a restricted data element not available to public users of PPMI data. The PPMI Data Access Committee approved use of the α-synuclein SAA and DaTscan results for participants. Statistical analysis codes used to perform the analyses in this article are shared on Zenodo (10.5281/zenodo.14047599). Information about the PPMI protocol can be found on protocols.io: https://doi.org/10.17504/protocols.io.n92ldmw6ol5b/v2.




